Professional Documents
Culture Documents
Summary of All Anemia
Uploaded by
benlarsena93%(14)93% found this document useful (14 votes)
5K views2 pagessummary_of_all anemia
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOC or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentsummary_of_all anemia
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC or read online from Scribd
93%(14)93% found this document useful (14 votes)
5K views2 pagesSummary of All Anemia
Uploaded by
benlarsenasummary_of_all anemia
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC or read online from Scribd
You are on page 1of 2
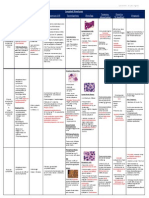
Hemolytic Anemias
Type Type of Mutation/ Cause Pathogenesis Clinical picture and findings
hemolysis
Sickle cell Extravascular Point mutation in beta-globin chain-glu On deoxygenation-polymerizaton and reversible Homozygous: severe anemia, HCT 18-30%,
anemia hemolysis replaced by val- S Hb sickling, continued exposure-ireversible – reticulocytosis, hyper-bilirubinemia, crisis
Can be homozygous- all chains have mononuclear phagocytosis & microvascular Hetero: asymptomatic until exposure to severe
mutation or heterozygous- abt half obstructions. hypoxia
chains have mutation Hypospleenism in adults, spleenomegaly in
Particularly predisposed with salmonella children- infections.
osteomyelitis
Thalasemia intravascular α- thalasemia: 4 genes on ch 16 Excessive β- & γ- chains- form stable tetramers Major:
hemolysis deletions- 1- silent carries, 4- hydrops (Hb H, Hb bart) low damage, ineffective in O2 Microcytic hypochromic + poikilocytosis
fetalis, 3- disease delivery Failure of normal development
β- thalasemia: 2 genes on ch 11 Decrease in β-chains – decreased Hb Skeletal deformities, reticulocytosis.
point mutations on certain regions of More imp: increase in free α-chains that aggregate, Iron overload – leads to cardiac failure (need
gene: in promoter & unsplicing region form inclusion in RBC (reduce plasticity of RBC, iron chelators)
of intron- β+ (reduced β-chains) becomes more susceptible to mononuclear Minor
In exon & splicing region of intron- β0 phagocytosis) destruction also of blasts – Mild microcytic hypochromic
(absence of β-chains) ineffective erythropoisis - iron overload Normal life expectancy
G6PD Intra- & extra- G6PD gene on ch X forms G6PD Exposure to drugs, toxins or infections increases H No symptoms unless exposure oxidative injury.
deficiency vascular enzyme that regenerates GSH after its peroxide so increases oxidation of GSH to GSSG, In males severe oxidant injury
hemolysis oxidation GSH regeneration is impaired so – accum. of H In females asymptomatic
Mutation causes more rapid decay of peroxide which denatures Hb. This ppts causing Heinz bodies (precipitated Hb) and bite cells
enzyme (A- variant) IVhemolysis and also EVhemolysis in spleen (phagocytosis by splenic phagocytes)
Paroxysmal intravascular Acquired membrane defect secondary PIGA deficient BM cells are present in normal PIG-tailed proteins (3 that prevent activation of
nocturnal hemolysis to a mutation that involves myeloid individuals, when there is immune-mediated complement on normal RBC’s) cannot be
hemoglobinuria stem cells. Mutation in X-linked PIGA destruction or suppression of BM cells by expressed so RBC sensitive to lytic activity of
(synthesis of intramembranous recognition of sp. PIG-tailed antigens the PIGA complement, also not expressed on granulocytes
glycoprotein anchor – PIG) deficient cells do not express the targets and so and platelets – susceptibility to infections and
escape the attack and replace BM IVthrombosis
Immuno- Warm: IgG or (rarely) IgA active at 37 Opsonization by IgG and subsequent phagocytosis +ve direct/ indirect coombs test for both
hemolytic deg. 60% idiopathic and 40 % by splenic macrophages. Attempts of phagocytosis Chronic mild anemia with moderate
anemia underlying disease (SLE) or drugs (a- leads to injured bits of CM – decreased SA:V ratio spleenomegaly
methyl dopa, penicillin) – spheroidal cells – sequestration
Extravascular
Cold: IgM binds to CM below 30 deg. Complement most active at 37 deg. so no Acute during recovery from Mycoplasma
hemolysis
(in distal parts) IVhemolysis, when cells go to warmer regions pneumonia and infectious mononucleosis or
IgM is not well bound but leaves behind C3b chronic resulting in transient mild anemia with
opsonin causing phagocytosis by kupffer cells often Raynaud phenomenon
(EV)
Resulting from Intravascular Valve prostheses and microangiopathic Valves cause abnormal pressure gradient and Burr, hemlet and triangle cells
trauma hemolysis (caused by DIC, malignant HT, SLE or turbulent blood flow, and RBCs are squeezed thru
disseminated cancer) narrowed vessels, results in mechanical damage in
both
malaria Intravascular Plasmodium vivax, malariea, ovale & After infecting hepatocytes, merozites infect Spikes of shaking, chills and fever at intervals
hemolysis falciparum (most fatal causing cerebral erythrocytes forming trophozites (characteristic to which coincide with merozite release from RBC.
malaria and blackwater fever) each specie) which divides giving rise to new Brown discoloration of spleen, liver, lymph
merozites that destroy RBCs upon escape nodes & BM, massive spleenomegaly
(hyperplasia of mononuclear phagocytes)
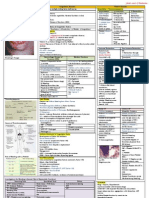
Anemias of Diminished Erythropoiesis
Type Cause Pathogenesis Clinical picture Diagnosis
Iron Deficiency Low dietary intake (rare), Starts with decline in serum ferretin and Mostly asymptomatic with weakness Low: Hb, HCT, MCV, serum
malabsorption (e.g. gastrectomy), stainable amounts of iron in BM. Followed and pallor in severe cases. Long-term ferretin, iron levels, transferring
increased demands (pregnancy, by decrease in circulating iron and rise in severs anemia – thinning, flattening saturation, microcytic hypochromic
infants), chronic blood loss (from transferrin iron-binding capacity – impact and spooning of fingernails, pica, RBCs. HIGH total iron-binding
GIT – peptic ulcer, hemorrhoids - on Hb, myoglobin and other iron increase in platelet count. Sometimes capacity
or female genital tract) compounds. When more severe – impaired develops plummer-vinson syndrome
work performance, brain function and
immunocompetence
Anemia of Occurs in chronic microbial Sequestration of iron from cells from the __ Normocytic normochromic, or
chronic disease infections (osteomyelitis, storage compartment (mononuclear hypocytic hypochromic, low serum
bacterial endocarditis, lung phagocyte storage pool) and suppression of Treatment: iron, (similar to iron deficiency)
abcess), chronic immune erythropoiesis due to inflammatory erythropoietin administration may but: Increased storage iron in
disorders (rheumatoid arthritis, mediators (IL-1, TNF, interferon-alpha) that improve but only treatment of marrow macrophages, increased
regional enteritis) and neoplasms are released from the underlying disease. underlying condition is reliable serum ferretin, decreased total iron-
(Hodgkin, lung & breast cancer) binding capacity
Megaloblastic Deficiency of folic acid from vit B12 required for regeneration of Non-specific symptoms relating to Smear of peripheral blood and bone
anemia poor diet or increased metabolic tetrahydrofolate, Folic acid provides pancytopenia (weakness, pallor, easy marrow. To differentiate between
needs. Or inhibition of folate tetrahydrofolate (carrier of a carbon group) fatigability, petechia, easy infection). vit B12 – serum folate and vit B12
metabolism by methotrexate, so both required for DNA synthesis. Alimentary tract related symptoms are levels and RBC folate levels
acidic foods & beans, phenytoin. Deficiency – delay in nuclear maturation common e.g. sore tonge (rapid dividing Low serum vit B12 levels, normal
pernicious anemia Impaired and cell division of erythroid precursors GIT cells). In vit B12 deficiency only or elevated folate, histamine-fast
absorption of vitamin B12 by: producing megaloblasts (also granulocyte neurological symptoms may take place gastric achlohydria, anti IF
*malabsorption (lack of vit B12 precursors produce giant megakaryocytes) e.g. symmetric numbness, tingling, antibodies, megaloblastic anemia
uncommon), *autoimmune some megaloblasts have very defective burning in feet or hands, unsteadiness findings, leucopenia, schilling test
reaction against parietal cells or DNA that they undergo apoptosis in BM, of gait, loss of position sense (unable to absorb an oral dose of vit
IF, others give mature RBC’s but output is *Cellular morphology: hypercellular B12 but when administered with IF
*surgery and gut disorders diminished. The enlarged RBCs are prone BM, nuclear-cytoplasmic asynchrony, absorption takes place)
(gastrectomy regional enteritis) to premature destruction by mononuclear megaloblasts – delicate finely
or *aging (gastric atrophy & phagocyte system leading to accumulation reticulated nuclear chromatin,
achlohydria) of iron (in mononuclear phagocytes) megakaryocytes – bizarre multi-lobed
nulei
Aplastic Suppression of multipotent Autoreactive T-cells (patients respond to Symptoms relating to pancytopenia Differential diagnosis to
myeloid stem cells caused by: immunosuppressive therapy aimed at T (weakness pallor, dyspnea, differentiate between it and
*idiopathic, *myelotoxic agents cells) where viral antigens, drug-drived petechiae,frequent persistant minor myelophthisic (or other
(irradiation or myelotoxic druds), haptens and genetic damage create infections or sudden onset of chills or pancytopenias) – hypocellular BM
*drugs and chemicals neoantigens within stem cells that are fever) owing to stem cell failure
(antineoplastic drugs, benzene, targets for autoreactive T cells Normocytic, normochromic, *Treatment: BM transplantation v.
chloramphenicol, or sensitivity to sometimes slight macrocytic. effective in patients nontransfused
sulfonamides, phenylbutazone Reticulocytosis is absent and younger than 40. other patients
etc.), *viral infection Spleenomegaly is absent – immunosuppressive therapy
Myelophthisic Associated with metastasis Bone marrow failure caused by extensive Anemia and thrombocytopenia Peripheral blood smear shows
arising from breast, lung, replacement of BM by tumors or other (pancytopenia) immature RBCs (teardrops) ,
prostate or thyroid 1ry lesion or lesions slightly elevated WBC count,
with myelofibrosis leukoerythroblastosis
You might also like
- AnemiasDocument2 pagesAnemiasdoktorcoop100% (2)
- Pathology Finals Reviewer on WBCs, Lymph Nodes, Spleen & ThymusDocument5 pagesPathology Finals Reviewer on WBCs, Lymph Nodes, Spleen & Thymusangel_sagun_1No ratings yet
- WBC Neoplasms Review - PathologyDocument6 pagesWBC Neoplasms Review - Pathologylas100% (6)
- Approach To Diagnosis of Haemolytic AnaemiasDocument2 pagesApproach To Diagnosis of Haemolytic AnaemiasGerardLumNo ratings yet
- Leukocytes Benign DisordersDocument3 pagesLeukocytes Benign DisordersGerardLum100% (3)
- Anti-Coagulants, Anti-Platelets, FibrinolyticsDocument1 pageAnti-Coagulants, Anti-Platelets, FibrinolyticsGerardLum100% (1)
- Fast Facts: Measurable Residual Disease: A clearer picture for treatment decisionsFrom EverandFast Facts: Measurable Residual Disease: A clearer picture for treatment decisionsNo ratings yet
- Hemostasis and Thrombosis: Practical Guidelines in Clinical ManagementFrom EverandHemostasis and Thrombosis: Practical Guidelines in Clinical ManagementHussain I. SabaNo ratings yet
- Fast Facts: Myelodysplastic Syndromes: Determining risk, tailoring therapy, supporting patientsFrom EverandFast Facts: Myelodysplastic Syndromes: Determining risk, tailoring therapy, supporting patientsNo ratings yet
- Acute and Chronic LeukemiasDocument3 pagesAcute and Chronic Leukemiaskaku100% (2)
- Hematologic DisordersDocument7 pagesHematologic DisordersCernan Oliveros100% (2)
- Hematologic Diseases OverviewDocument11 pagesHematologic Diseases OverviewPerrilyn Perey100% (2)
- Lab Values: An Easy Guide to Learn Everything You Need to Know About Laboratory Medicine and Its Relevance in Diagnosing DiseaseFrom EverandLab Values: An Easy Guide to Learn Everything You Need to Know About Laboratory Medicine and Its Relevance in Diagnosing DiseaseRating: 5 out of 5 stars5/5 (2)
- Blood FilmDocument2 pagesBlood FilmGerardLum100% (1)
- Hematology Tables Morphology of RBCsDocument5 pagesHematology Tables Morphology of RBCsGlydenne Glaire Poncardas GayamNo ratings yet
- Bone Marrow FailureDocument2 pagesBone Marrow FailureGerardLum100% (1)
- Essentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationFrom EverandEssentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationRating: 5 out of 5 stars5/5 (1)
- Fast Facts: Peripheral T-cell Lymphoma: Unraveling the complexities of diagnosis and managementFrom EverandFast Facts: Peripheral T-cell Lymphoma: Unraveling the complexities of diagnosis and managementNo ratings yet
- Hemophilia and Von Willebrand Disease: Factor VIII and Von Willebrand FactorFrom EverandHemophilia and Von Willebrand Disease: Factor VIII and Von Willebrand FactorNo ratings yet
- The Complete Guide on Anemia: Learn Anemia Symptoms, Anemia Causes, and Anemia Treatments. Anemia types covered in full details: Iron-deficiency, Microcytic, Autoimmune Hemolytic, Sideroblastic, and Normocytic AnemiaFrom EverandThe Complete Guide on Anemia: Learn Anemia Symptoms, Anemia Causes, and Anemia Treatments. Anemia types covered in full details: Iron-deficiency, Microcytic, Autoimmune Hemolytic, Sideroblastic, and Normocytic AnemiaRating: 3.5 out of 5 stars3.5/5 (9)
- All Objectives HematologyDocument45 pagesAll Objectives HematologyNursing200980% (5)
- Full Blood PictureDocument1 pageFull Blood PictureGerardLumNo ratings yet
- 1 Blood SmearDocument59 pages1 Blood SmearGabi Tim100% (1)
- MUST To KNOW in Immunohematology Blood BankingDocument42 pagesMUST To KNOW in Immunohematology Blood BankingMerhella Amor Suerte MendozaNo ratings yet
- HAEMOPOIESISDocument6 pagesHAEMOPOIESISDiyana ZahariNo ratings yet
- Endocrine Pathology p17-32Document16 pagesEndocrine Pathology p17-32zeroun24No ratings yet
- Acquired Bleeding DisordersDocument1 pageAcquired Bleeding DisordersGerardLumNo ratings yet
- Differences in Sickle Cell Disease VariantsDocument3 pagesDifferences in Sickle Cell Disease VariantsMeevie Toledo0% (1)
- MUST To KNOW in HematologyDocument45 pagesMUST To KNOW in HematologyDen100% (4)
- Hematology 2 LaboratoryDocument11 pagesHematology 2 LaboratoryChristine BadilloNo ratings yet
- Role of Blood Vessels in Hemostasis: Villa, M.D. MLS 4CDocument10 pagesRole of Blood Vessels in Hemostasis: Villa, M.D. MLS 4CMarianne Dennesse100% (1)
- Hematologic Disorders NotesDocument19 pagesHematologic Disorders Notesmikkagreen95% (22)
- 22 - ImmunohematologyDocument6 pages22 - Immunohematologyhamadadodo7No ratings yet
- Acute Complications of Diabetes MellitusDocument1 pageAcute Complications of Diabetes MellitusGerardLum100% (1)
- AnemiaDocument1 pageAnemiaindriyanti natasya ayu utami kottenNo ratings yet
- Gi Pathology - Block 3 ReviewDocument34 pagesGi Pathology - Block 3 ReviewMatt McGlothlin100% (1)
- Hemotology Pathology PDFDocument15 pagesHemotology Pathology PDFMorgan PeggNo ratings yet
- MUST To KNOW in HematologyDocument40 pagesMUST To KNOW in HematologyPatricia Yutuc90% (10)
- Clin Path Lab 6 UrinalysisDocument5 pagesClin Path Lab 6 Urinalysisapi-3743217100% (6)
- Hema Notes (Lec)Document50 pagesHema Notes (Lec)Anonymous 0zrCNQNo ratings yet
- HematologyDocument5 pagesHematologyIvy Jan OcateNo ratings yet
- Hematology NotesDocument17 pagesHematology NotesEly Sibayan100% (2)
- HematologyDocument42 pagesHematologyadaako100% (8)
- RBC DisordersDocument70 pagesRBC DisordersNdor Baribolo100% (1)
- Hematology ReviewerDocument4 pagesHematology ReviewerAbigail Puno100% (1)
- Decreased Levels of Iron by Diet or Hemorrhage Impaired Heme SynthesisDocument8 pagesDecreased Levels of Iron by Diet or Hemorrhage Impaired Heme SynthesisSamuel RothschildNo ratings yet
- Common Diseases Review - Community MedicineDocument15 pagesCommon Diseases Review - Community Medicinelas85% (13)
- Coagulation CascadeDocument5 pagesCoagulation Cascadepieterinpretoria391100% (1)
- AnemiaDocument10 pagesAnemiaBia Payawal100% (2)
- NUR 102 - Chapter 14 Fluid and ElectrolytesDocument32 pagesNUR 102 - Chapter 14 Fluid and ElectrolytesIanna J. L. Pedrosa100% (1)
- COX-2 Inhibitors: Mechanisms and ExamplesDocument16 pagesCOX-2 Inhibitors: Mechanisms and ExamplesYousab MKNo ratings yet
- Pelvic Traction SlingDocument3 pagesPelvic Traction SlingArturo Jr Garces RNNo ratings yet
- Major Clinical Features of Genital Ulcers ChecklistDocument1 pageMajor Clinical Features of Genital Ulcers Checklistgdudex118811No ratings yet
- Rotator Cuff Injuries: Symptoms, Causes and TreatmentDocument2 pagesRotator Cuff Injuries: Symptoms, Causes and TreatmentMinnie Hubalde-LoprettiNo ratings yet
- Artículo 7000 PalabrasDocument8 pagesArtículo 7000 PalabrasEmalaith BlackburnNo ratings yet
- Health 9 2nd Activity 1 Philippine Drug ScenarioDocument2 pagesHealth 9 2nd Activity 1 Philippine Drug ScenarioRyan BersaminNo ratings yet
- Hematology NCLEX MCQDocument24 pagesHematology NCLEX MCQKo Ye100% (2)
- Teeter FitSpine X1 Inversion Table Owners ManualDocument6 pagesTeeter FitSpine X1 Inversion Table Owners ManualhawkjohnNo ratings yet
- MedVantage - Fellowship Program in Diabetes MillitusDocument2 pagesMedVantage - Fellowship Program in Diabetes MillitusmedvantageNo ratings yet
- Test Bank For Evidence Based Practice in Nursing Healthcare 4th EditionDocument36 pagesTest Bank For Evidence Based Practice in Nursing Healthcare 4th Editiontyphous.madrierdvfzai100% (47)
- Appendix I: PROFORMADocument6 pagesAppendix I: PROFORMAVishwas NayakNo ratings yet
- Guaifenesin: What Is in This LeafletDocument6 pagesGuaifenesin: What Is in This LeafletKate Felongco CambelNo ratings yet
- University of Delaware College of Health and Nursing Sciences - Hepatitis B Vaccine Declination Form Students)Document2 pagesUniversity of Delaware College of Health and Nursing Sciences - Hepatitis B Vaccine Declination Form Students)DonnaNo ratings yet
- HargaDocument1,176 pagesHargaRSUD SOLOKNo ratings yet
- Insertion and Management of Peripheral Intravenous Cannulae in Western Australian Healthcare Facilities PolicyDocument22 pagesInsertion and Management of Peripheral Intravenous Cannulae in Western Australian Healthcare Facilities PolicyChristian RSNo ratings yet
- Management of Intravascular Devices To Prevent Infection: LinicalDocument5 pagesManagement of Intravascular Devices To Prevent Infection: LinicalCristianMedranoVargasNo ratings yet
- Seer Survival Mono LowresDocument286 pagesSeer Survival Mono LowresPadmaswari HarumiNo ratings yet
- Ef310 Unit 08 Client Assessment Matrix Fitt Pros-3Document6 pagesEf310 Unit 08 Client Assessment Matrix Fitt Pros-3api-295146168No ratings yet
- Care With Git PTDocument16 pagesCare With Git PTHafidz Ma'rufNo ratings yet
- Hydrocephalus AND Neural Tube DefectDocument7 pagesHydrocephalus AND Neural Tube DefectTherese ArellanoNo ratings yet
- Perez TMC-213 Module-2Document4 pagesPerez TMC-213 Module-2NISHA MIKLE MACULNo ratings yet
- HIV Natural History StagesDocument10 pagesHIV Natural History StagesNirav Sharma100% (1)
- Anti Thyroid DrugsDocument22 pagesAnti Thyroid DrugsShahid HameedNo ratings yet
- Futility CasesDocument2 pagesFutility CasesBindashboy0No ratings yet
- What Is The Appendix?: Symptoms of AppendicitisDocument6 pagesWhat Is The Appendix?: Symptoms of AppendicitisVonn Bryan CalumiaNo ratings yet
- ATI RN Mental Health Online Practice BDocument38 pagesATI RN Mental Health Online Practice Bseansdrew2No ratings yet
- DR Walsh Autism Ocd Pandas Depression MethylationDocument6 pagesDR Walsh Autism Ocd Pandas Depression MethylationAnupama PouloseNo ratings yet
- Pharmacovigilance 1Document28 pagesPharmacovigilance 1Gideon AdurojaNo ratings yet
- Angle-Closure Glaucoma - UpToDateDocument9 pagesAngle-Closure Glaucoma - UpToDateElaine June FielNo ratings yet