Professional Documents
Culture Documents
03 Haber and Contact Processes
Uploaded by
api-235688447Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
03 Haber and Contact Processes
Uploaded by
api-235688447Copyright:
Available Formats
HABER AND CONTACT PROCESSES: APPLYING CONCEPTS BY DANIELLE POSEN
Directions: Here is your chance to apply anything and everything from topic 7 to two specific industrial processes. Your assignment is to write a prose-style report that looks at both of these processes in depth, including material that is relevant to the assessment statements from this topic. It is possible (and perhaps likely) that you wont find information pertinent to each assessment statement, so dont get overly consumed with finding every detail.
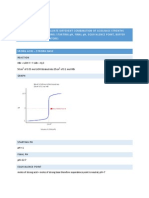
Both the Haber and Contact Process are examples of Dyanamic Equilibrium Reactions. The haber process is used in the production of Ammonia which increases the growth of plants. It was even assumed that it sustained one third of the worlds population. The equation is: N2 + 3 H2 < 2 NH3 (H = 92.4 kJmol1) The forward reaction is exothermic therefore to obtain the highest yield of ammonia a low temperature would be needed as exothermic reactions produce heat. In terms of pressure the greatest yield would be achieved with a high pressure as the forward reaction produces the least amount of moles of gas. These conditions are, however, unideal, because the production of high pressure pipes is expensive and having a low temperature would decrease the rate at which the reaction reaches equilibrium, therefore it would be inefficient. Rather a temperature of between 350 C to 450 C is used while the pressure is about 150-220 atmospheres. This produces 15% yield of ammonia. The catalyst used in this reaction is iron metal. It is used in its powdered form as to increase surface area. The catalyst decreases the activation energy therefore particles can overcome the activation energy more easily. A catalyst does not shift equilibrium as both the forward and reverse reactions are affected equally. The Kc constant expression for the haber process is:
The reverse reaction is position as the numerator while the forward reaction reactants are placed as the denominator the brackets represent concentration of the molecule. The contact process if used in the manufacturing of sulfuric acids. Sulfuric acid is used to make a phosphate fertilizer. The reactions shows the conversion of sulfur dioxide to sulfur trioxide:
The ideal conditions to produce the highest yield would be to lower the temperature and increase pressure the same as the Haber Process. However, because the manufacturing of high pressure resistant pipes is extremely expensive a lower pressure is used of 1-2 atmospheres with a temperature of 400 C to 450 C. The catalyst V2O5 is used in the contact process, it reduces the activation energy therefore increasing the
speed at which equilibrium is reached. A catalyst will not affect the position of equilibrium as it affects both the forward and reverse reaction equally. The Kc constant expression for the contact process is:
You might also like
- TitrationsDocument4 pagesTitrationsapi-235688447No ratings yet
- 01 Phet PH ScaleDocument2 pages01 Phet PH Scaleapi-235688447No ratings yet
- Venn Diagram of The Different Definitions For Acids and BasesDocument1 pageVenn Diagram of The Different Definitions For Acids and Basesapi-235688447No ratings yet
- Acids and Metals NewDocument4 pagesAcids and Metals Newapi-235688447No ratings yet
- 18 5Document3 pages18 5api-235688447No ratings yet
- 18.4 Acid-Base Titrations (HL)Document2 pages18.4 Acid-Base Titrations (HL)api-235688447No ratings yet
- 18.3 Salt Hydrolysis (HL)Document1 page18.3 Salt Hydrolysis (HL)api-235688447No ratings yet
- 8.3 Strong and Weak Acids & BasesDocument1 page8.3 Strong and Weak Acids & Basesapi-235688447No ratings yet
- Vapour Pressure LabDocument3 pagesVapour Pressure Labapi-235688447No ratings yet
- 18 2 BufferDocument2 pages18 2 Bufferapi-235688447No ratings yet
- 8.4 The PH ScaleDocument1 page8.4 The PH Scaleapi-235688447No ratings yet
- 18.1: Calculations Involving Acids & Bases (HL)Document6 pages18.1: Calculations Involving Acids & Bases (HL)api-235688447No ratings yet
- 8.2 Properties of Acids and BasesDocument1 page8.2 Properties of Acids and Basesapi-235688447No ratings yet
- 8 1Document3 pages8 1api-235688447No ratings yet
- 01 Phet Reversible ReactionsDocument2 pages01 Phet Reversible Reactionsapi-235688447100% (1)
- Le Chateliers Principle Lab DanielleDocument4 pagesLe Chateliers Principle Lab Danielleapi-235688447No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Supersonic Channel Flow With Shocks PDFDocument9 pagesSupersonic Channel Flow With Shocks PDFPrince Israel EboigbeNo ratings yet
- Phys532 Pset 2Document2 pagesPhys532 Pset 2waskhezNo ratings yet
- Chrom 4.3 - Sielc ArticleDocument5 pagesChrom 4.3 - Sielc ArticleKhadija HameedNo ratings yet
- Key Comparison CCQM-K34.2 Assay of Potassium Hydrogen PhthalateDocument12 pagesKey Comparison CCQM-K34.2 Assay of Potassium Hydrogen PhthalatemariassyNo ratings yet
- Foundations in Microbiology 9th Edition by Talaro and Chess Solution ManualDocument3 pagesFoundations in Microbiology 9th Edition by Talaro and Chess Solution ManualcamriidNo ratings yet
- Use of Agro-Waste-Based AdsorbentsDocument15 pagesUse of Agro-Waste-Based AdsorbentsAHMEDNo ratings yet
- Emerging Solar Cell PDFDocument23 pagesEmerging Solar Cell PDFJaffar LoneNo ratings yet
- 12 - Chapter 2 PDFDocument27 pages12 - Chapter 2 PDFMohd SalmanNo ratings yet
- Hydrogen Attack PDFDocument11 pagesHydrogen Attack PDFChanoNo ratings yet
- Chapter 4 Isothermal Reactor Design (Class Discussion)Document6 pagesChapter 4 Isothermal Reactor Design (Class Discussion)FakhrulShahrilEzanieNo ratings yet
- Met Glossary 1963 PDFDocument327 pagesMet Glossary 1963 PDFjavier albaNo ratings yet
- Report AdaniDocument38 pagesReport AdaniMazumJainNo ratings yet
- Two Way 4D Printing - A Review On The Reversibility of 3D Printed Shape Memory MaterialsDocument12 pagesTwo Way 4D Printing - A Review On The Reversibility of 3D Printed Shape Memory MaterialsEdgar ApitanaNo ratings yet
- Light Properties LectureDocument49 pagesLight Properties LecturemkanwarsNo ratings yet
- Unit 3: Lesson 2 Interactions of LightDocument16 pagesUnit 3: Lesson 2 Interactions of LightAbeer Alnajjar عبير النجارNo ratings yet
- Bgas gr-2 W AnswerDocument33 pagesBgas gr-2 W AnswerShaji Thomas100% (4)
- As Week 3 Q2Document5 pagesAs Week 3 Q2Elaine MagpatagNo ratings yet
- CH 9. Ionic Equilibrium (Chem +1)Document43 pagesCH 9. Ionic Equilibrium (Chem +1)nitinNo ratings yet
- Rotary Evaporators Evaporation SolutionsDocument8 pagesRotary Evaporators Evaporation SolutionsFungusface PathogenNo ratings yet
- 4.2 Laboratory Report Acids and BasesDocument5 pages4.2 Laboratory Report Acids and Basesalejandro pederioNo ratings yet
- Ion Exchange Resin.F2005Document3 pagesIon Exchange Resin.F2005Almira Kaye CuadraNo ratings yet
- Chem 11 Final Exam Review KeyDocument12 pagesChem 11 Final Exam Review Keyboriana72No ratings yet
- 1 s2.0 S0017931008001440 MainDocument9 pages1 s2.0 S0017931008001440 Maingen liNo ratings yet
- Assignment 1Document3 pagesAssignment 1Prakash Singh100% (2)
- Electrolysis: Edited by Janis Kleperis and Vladimir LinkovDocument300 pagesElectrolysis: Edited by Janis Kleperis and Vladimir LinkovoNo ratings yet
- Effective Laboratory Coreflood TestsDocument15 pagesEffective Laboratory Coreflood TestsJj WeetlandNo ratings yet
- Module 3 and 4Document8 pagesModule 3 and 4Thea Louise GarciaNo ratings yet
- 2006 - Kamal and Mohamad Combustion in Porous MediaDocument22 pages2006 - Kamal and Mohamad Combustion in Porous Mediavivek joshiNo ratings yet
- Chapter 4electric Fields in MatterDocument51 pagesChapter 4electric Fields in MatterAxel Coronado PopperNo ratings yet
- Cy1104 - Engineering Chemistry Unit - 4 Fuels and Combustion Lecture PlanDocument22 pagesCy1104 - Engineering Chemistry Unit - 4 Fuels and Combustion Lecture PlanBeuna.No ratings yet