Professional Documents
Culture Documents
Atomic Volumes of The Elements
Uploaded by
skruzerOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Volumes of The Elements
Uploaded by
skruzerCopyright:
Available Formats
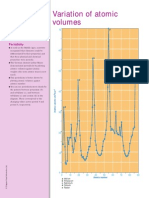
ELEM ENT S AND CO M PO UNDS
Atomic volume
G The atomic volumei sthe volume of
one moleof the atomsof an element.
I t can be found by di vi di ng the atomic
massof one mole of atomsby the
densi ty of the element:
A to m i c v o lu m e = A to m i c m a ss
D e n si ty
G Si nce there are 6.023 x 10
23
atomsper
mole of atoms, i t would seem possi ble
to use the atomi c volume to calculate
the volume of a si ngle atom, and thus
i tsradi us. However there are two
problemswi th doi ng thi s. Fi rst, the
state of an element, and therefore i ts
densi ty, changeswi th temperature and
pressure. Second, usi ng the atomi c
volume to calculate the volume of a
si ngle atom assumesthat an element
consi stsof atomsthat are not bonded
to each other. Thi si strue only of the
group 8elements( noble gases) . For
these reasons, i t i snot possi ble to
consi der the volume of an atom i n
i solati on, but only aspart of the
structure of an element.
G I n general, atomi c volume i ncreases
down a group. Acrossa period, i t
decreasesand then i ncreases.
G The di agram hi ghli ghtsthe element
wi th the hi ghest atomi c volume i n the
fi rst si x peri ods( mi nusthe lanthanide
series) .
E le m e n ts wi th p e a k a to m i c
v o lu m e s
H e H e li u m
K P o ta ssi u m
R b R u b i d i u m
C s C e si u m
R n R a d o n
Atomic volumes of the
elements
atomic mass
atomic volume
density
element
group
group 8
lanthanide series
mole
noble gases
period
Key words
33
D
i
a
g
r
a
m
V
i
s
u
a
l
I
n
f
o
r
m
a
t
i
o
n
L
t
d
.
1
3
.
5
1
0
.
9
9
.
6
8
.
9
8
.
5
8
.
6
9
.
1
1
0
.
2
1
4
.
8
1
7
.
2
1
8
.
3
2
1
.
4
5
0
.
5
1
4
.
2
1
0
.
9
9
.
4
8
.
1
8
.
3
8
.
8
1
0
.
3
1
3
.
0
1
5
.
8
1
6
.
4
1
8
.
2
2
0
.
4
2
5
.
6
4
2
.
9
Z
r
N
b
M
o
T
c
R
u
R
h
P
d
A
g
C
d
I
n
S
n
S
b
T
e
I
X
e
H
f
T
a
W
R
e
O
s
I
r
P
t
A
u
H
g
T
l
P
b
B
i
P
o
A
t
R
n
7
1
.
0
3
9
.
2
5
5
.
7
3
4
.
0
1
6
.
1
R
b
S
r
Y
C
s
B
a
1
0
.
6
8
.
9
7
.
3
7
.
4
7
.
1
6
.
6
6
.
6
7
.
1
9
.
2
1
1
.
8
1
3
.
3
1
3
.
1
1
6
.
5
2
5
.
6
3
2
.
2
T
i
V
C
r
M
n
F
e
C
o
N
i
C
u
Z
n
G
a
G
e
A
s
S
e
B
r
K
r
4
4
.
9
2
6
.
0
1
4
.
7
K
C
a
S
c
4
.
3
5
.
4
1
7
.
3
1
4
.
0
1
7
.
1
1
6
.
8
B
C
N
O
F
N
e
2
3
.
7
1
4
.
0
N
a
M
g
1
3
.
0
4
.
9
L
i
B
e
1
0
.
0
1
1
.
6
1
6
.
9
1
5
.
5
1
8
.
7
2
4
.
2
A
l
S
i
P
S
C
l
A
r
3
1
.
8
H
e
1
4
.
1
H
1
56 4 3 2
L
a
2
2
.
6
8
.
5
2
2
.
2
3
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Solar SystemDocument1 pageSolar SystemskruzerNo ratings yet
- Variation of Boiling PointsDocument1 pageVariation of Boiling PointsskruzerNo ratings yet
- Variation of Atomic NumbersDocument1 pageVariation of Atomic NumbersskruzerNo ratings yet
- Variation of Melting PointsDocument1 pageVariation of Melting PointsskruzerNo ratings yet
- The Periodic TableDocument1 pageThe Periodic Tableskruzer100% (1)
- Planetary Density, Size, and AtmosphereDocument1 pagePlanetary Density, Size, and AtmosphereskruzerNo ratings yet
- Structure of Some Ionic CrystalsDocument1 pageStructure of Some Ionic CrystalsskruzerNo ratings yet
- The MoleDocument1 pageThe MoleskruzerNo ratings yet
- Si UnitsDocument12 pagesSi UnitsskruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Planet CompositionDocument1 pagePlanet CompositionskruzerNo ratings yet
- Periodic Table With Masses and NumbersDocument1 pagePeriodic Table With Masses and NumbersskruzerNo ratings yet
- Organizing The ElementsDocument1 pageOrganizing The ElementsskruzerNo ratings yet
- Melting Points of The Elements CDocument1 pageMelting Points of The Elements CskruzerNo ratings yet
- Luminescence Atomic StructureDocument1 pageLuminescence Atomic StructureskruzerNo ratings yet
- Measuring The Charge On The ElectronDocument1 pageMeasuring The Charge On The ElectronskruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Calculate The Molecular Mass of CompoundsDocument1 pageCalculate The Molecular Mass of CompoundsskruzerNo ratings yet
- Investigating The Electron 1Document1 pageInvestigating The Electron 1skruzerNo ratings yet
- Determination of Avogadro's ConstantDocument1 pageDetermination of Avogadro's ConstantskruzerNo ratings yet
- Energy Levels HydrogenDocument1 pageEnergy Levels HydrogenskruzerNo ratings yet
- Geiger and Marsden's ApparatusDocument1 pageGeiger and Marsden's ApparatusskruzerNo ratings yet
- Cathode Ray OscilloscopeDocument1 pageCathode Ray OscilloscopeskruzerNo ratings yet
- Crystal Structure of Metals Lattice Structure 0Document1 pageCrystal Structure of Metals Lattice Structure 0skruzerNo ratings yet
- Boiling Points of The Elements CDocument1 pageBoiling Points of The Elements CskruzerNo ratings yet
- Crystal Structure of Metals Efficient PackingDocument1 pageCrystal Structure of Metals Efficient PackingskruzerNo ratings yet
- Atomic StructureDocument1 pageAtomic StructureskruzerNo ratings yet
- Atomic MassDocument1 pageAtomic MassskruzerNo ratings yet
- Atomic Emission Spectrum HydrogenDocument1 pageAtomic Emission Spectrum HydrogenskruzerNo ratings yet