Professional Documents
Culture Documents
Determination of Avogadro's Constant
Uploaded by
skruzerOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Determination of Avogadro's Constant
Uploaded by
skruzerCopyright:
Available Formats
Determination of
Avogadros constant
AT O M I C ST RUCT URE
Defining Avogadros
constant
G Avogadros constant i sthe number of
parti clesi n a moleof a substance. I t
equals6.023 x 10
23
mol
-1
.
G I t i sF , the Faraday constant 96,500
coulombsper mole, the amount of
electri c charge of one mole of
electrons di vi ded by 1.60 x 10
-19
coulomb the charge on one electron
( expressed ase ) .
G Thus, the Avogadro constant, N , i s
gi ven by: N = F
e
or:
9 6 , 5 0 0 = 6 . 0 2 3 x 1 0
2 3
m o l
-1
1 . 6 0 x 1 0
-1 9
Determining the Constant
G The number of moleculesi n one mole
of substance can be determi ned by
usi ng electrochemi stry.
G Duri ng electrolysis, current ( electron
flow) over ti me i smeasured i n an
electrolyti c cell ( see di agram) . The
number of atomsi n a wei ghed sample
i sthen related to the current to
calculate Avogadro sconstant.
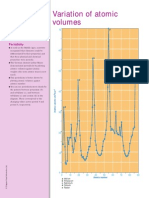
Illustrating the Procedure
G The di agram i llustratesthe electrolysi s
of copper sulfate. To calculate
Avogadro sconstant, the researcher
wei ghsthe rod to be used asthe
anodebefore submergi ng the two
copper rodsi n copper sulfate. She
then connectsthe rodsto a power
supply and an ammeter ( an
i nstrument used to measure electri c
current) . She measuresand records
the current at regular i ntervalsand
calculatesthe average amperage ( the
uni t of electri c current) . O nce she
turnsoff the current, she wei ghsthe
anode to see how much masswaslost.
Usi ng the fi guresfor anode masslost,
average current, and durati on of the
electrolysi s, she calculatesAvogadro s
constant.
anode
Avogadros
constant
electrolysis
Faraday constant
mole
Key words
20
A
+
Determination of Avogadros constant
a b
c
d
e
f
a p o we r su p p ly wi th a m m e te r
b rh e o sta t
c h a rd b o a rd o r wo o d e n e le c tro d e h o ld e r
d co p p e r ro d ca th o d e
e co p p e r ro d a n o d e
f co p p e r su lfa te so lu ti o n
D
i
a
g
r
a
m
V
i
s
u
a
l
I
n
f
o
r
m
a
t
i
o
n
L
t
d
.
You might also like
- Variation of Melting PointsDocument1 pageVariation of Melting PointsskruzerNo ratings yet
- The MoleDocument1 pageThe MoleskruzerNo ratings yet
- The Periodic TableDocument1 pageThe Periodic Tableskruzer100% (1)
- Variation of Boiling PointsDocument1 pageVariation of Boiling PointsskruzerNo ratings yet
- Variation of Atomic NumbersDocument1 pageVariation of Atomic NumbersskruzerNo ratings yet
- Structure of Some Ionic CrystalsDocument1 pageStructure of Some Ionic CrystalsskruzerNo ratings yet
- Solar SystemDocument1 pageSolar SystemskruzerNo ratings yet
- Organizing The ElementsDocument1 pageOrganizing The ElementsskruzerNo ratings yet
- Si UnitsDocument12 pagesSi UnitsskruzerNo ratings yet
- Planetary Density, Size, and AtmosphereDocument1 pagePlanetary Density, Size, and AtmosphereskruzerNo ratings yet
- Periodic Table With Masses and NumbersDocument1 pagePeriodic Table With Masses and NumbersskruzerNo ratings yet
- Melting Points of The Elements CDocument1 pageMelting Points of The Elements CskruzerNo ratings yet
- Planet CompositionDocument1 pagePlanet CompositionskruzerNo ratings yet
- Measuring The Charge On The ElectronDocument1 pageMeasuring The Charge On The ElectronskruzerNo ratings yet
- Luminescence Atomic StructureDocument1 pageLuminescence Atomic StructureskruzerNo ratings yet
- Crystal Structure of Metals Efficient PackingDocument1 pageCrystal Structure of Metals Efficient PackingskruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Energy Levels HydrogenDocument1 pageEnergy Levels HydrogenskruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Geiger and Marsden's ApparatusDocument1 pageGeiger and Marsden's ApparatusskruzerNo ratings yet
- Investigating The Electron 1Document1 pageInvestigating The Electron 1skruzerNo ratings yet
- Crystal Structure of Metals Lattice Structure 0Document1 pageCrystal Structure of Metals Lattice Structure 0skruzerNo ratings yet
- Atomic Volumes of The ElementsDocument1 pageAtomic Volumes of The ElementsskruzerNo ratings yet
- Cathode Ray OscilloscopeDocument1 pageCathode Ray OscilloscopeskruzerNo ratings yet
- Calculate The Molecular Mass of CompoundsDocument1 pageCalculate The Molecular Mass of CompoundsskruzerNo ratings yet
- Boiling Points of The Elements CDocument1 pageBoiling Points of The Elements CskruzerNo ratings yet
- Atomic MassDocument1 pageAtomic MassskruzerNo ratings yet
- Atomic StructureDocument1 pageAtomic StructureskruzerNo ratings yet
- Atomic Emission Spectrum HydrogenDocument1 pageAtomic Emission Spectrum HydrogenskruzerNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Strength of Material 2Document12 pagesStrength of Material 2mjdalenezi100% (1)
- Michelson Interferometer ArticleDocument3 pagesMichelson Interferometer ArticleWILLIAM IMMANUEL MARTINNo ratings yet
- Assignment 1 - MagnetismDocument3 pagesAssignment 1 - MagnetismAnanya SinghNo ratings yet
- Klinkenberg CorrectionDocument2 pagesKlinkenberg Correctionandmol5796No ratings yet
- Gsa NotesDocument4 pagesGsa NotesLeenaNo ratings yet
- Relativity VIC McqsDocument3 pagesRelativity VIC McqsLasnthaBandara75% (4)
- Still Water BM and SFDocument5 pagesStill Water BM and SFpothirajkalyanNo ratings yet
- Formula Sheet Class 12Document5 pagesFormula Sheet Class 12shikhary167100% (1)
- D. Myers - Surfaces, Interfaces and Colloids - Principles and ApplicationsDocument520 pagesD. Myers - Surfaces, Interfaces and Colloids - Principles and ApplicationsAmairanyta Hernandez Zarate100% (4)
- Phy122 Em1 2023Document1 pagePhy122 Em1 2023Resego lentsweNo ratings yet
- Jackson 4 13 Homework SolutionDocument3 pagesJackson 4 13 Homework SolutionHind Abu GhazlehNo ratings yet
- DynamicsDocument8 pagesDynamicsSurafel BehailuNo ratings yet
- Relay: Relay Is Basically A Magnetism Based Switch. It Consists of A Coil Through Which Current Passes and OnDocument9 pagesRelay: Relay Is Basically A Magnetism Based Switch. It Consists of A Coil Through Which Current Passes and OnAnonymous v5QjDW2eHxNo ratings yet
- Gorom Angelica Lirio R. Phys101l b8 E102 2q1819Document3 pagesGorom Angelica Lirio R. Phys101l b8 E102 2q1819Janno RafaelNo ratings yet
- Imperial College London Bsci/Msci Examination May 2016 Mph2 Mathematical MethodsDocument6 pagesImperial College London Bsci/Msci Examination May 2016 Mph2 Mathematical MethodsRoy VeseyNo ratings yet
- R 507Document1 pageR 507Fernando Cordova PardoNo ratings yet
- Internal Combustion Engines - H. B. Keswani - 2Document169 pagesInternal Combustion Engines - H. B. Keswani - 2Salman ShaxShax HeissNo ratings yet
- 03 Gas ReformatDocument29 pages03 Gas Reformatshanthiny75No ratings yet
- Passive Components (Compatibility Mode)Document102 pagesPassive Components (Compatibility Mode)gauravNo ratings yet
- Elevated Water Tank Design SpreadsheetDocument12 pagesElevated Water Tank Design SpreadsheetRuben Dario Posada B100% (3)
- An Improved Efficient Electric Bicycle System With The Power of Real-Time Information SharingDocument4 pagesAn Improved Efficient Electric Bicycle System With The Power of Real-Time Information SharingsubiNo ratings yet
- Ag Cu inDocument8 pagesAg Cu inReda TammamNo ratings yet
- A Comprehensive Method For Inspecting Cube Corner Prism Based On Shack-Hartmann Wavefront SensorDocument6 pagesA Comprehensive Method For Inspecting Cube Corner Prism Based On Shack-Hartmann Wavefront Sensormitra1006No ratings yet
- Rohini 42795977333Document3 pagesRohini 42795977333madesh1047No ratings yet
- POGIL Molecular GeometryDocument3 pagesPOGIL Molecular Geometryliza120750% (2)
- SSC JE Civil (2016) SET 4 Watermark - PDF 49Document11 pagesSSC JE Civil (2016) SET 4 Watermark - PDF 49Safikul HossainNo ratings yet
- Physics Practice Question Paper 1Document16 pagesPhysics Practice Question Paper 1Divyanshu SinghNo ratings yet
- Enthuse SRG TEST-8 FinalDocument26 pagesEnthuse SRG TEST-8 FinalremorevdrakeNo ratings yet
- 09-Advanced Transport PhenomenaDocument1 page09-Advanced Transport Phenomenasudhakar kNo ratings yet
- Notes Anatomy and PhysiologyDocument5 pagesNotes Anatomy and PhysiologyEllah MaeNo ratings yet