Professional Documents
Culture Documents
Variation of Boiling Points
Uploaded by
skruzerCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Variation of Boiling Points
Uploaded by
skruzerCopyright:
Available Formats
Variation of boiling
points
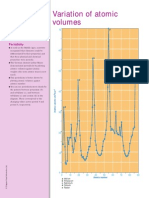
ELEM ENT S AND CO M PO UNDS
Variation of boiling point
G The majori ty of non-metalli c elements
are gasesat room temperature and
atmospheri c pressure. Most non-
metalli c elementshave si mple covalent
structuresand have very low boiling
points.
G Elementswi th metalli c and gi ant
covalent structureshave very hi gh
boi li ng poi nts( see di agram) . The
boi li ng poi ntsof transition metalsare
generally much hi gher than those of
the group 1and group 2metals.
boiling point
gas
group 1
group 2
transition metals
Key words
32
B
o
i
l
i
n
g
p
o
i
n
t
C
2 , 5 0 0
9 0
Atomic number
2 , 0 0 0
1 , 0 0 0
5 0 0
0
0 8 0 7 0 6 0 5 0 4 0 3 0 2 0 1 0
a
b
d c e
1 , 5 0 0
3 , 0 0 0
a C a rb o n
b S i li co n
c Va n a d i u m
d M o ly b d e n u m
e R h e n i u m
D
i
a
g
r
a
m
V
i
s
u
a
l
I
n
f
o
r
m
a
t
i
o
n
L
t
d
.
You might also like
- Solar SystemDocument1 pageSolar SystemskruzerNo ratings yet
- Variation of Melting PointsDocument1 pageVariation of Melting PointsskruzerNo ratings yet
- The MoleDocument1 pageThe MoleskruzerNo ratings yet
- The Periodic TableDocument1 pageThe Periodic Tableskruzer100% (1)
- Structure of Some Ionic CrystalsDocument1 pageStructure of Some Ionic CrystalsskruzerNo ratings yet
- Variation of Atomic NumbersDocument1 pageVariation of Atomic NumbersskruzerNo ratings yet
- Measuring The Charge On The ElectronDocument1 pageMeasuring The Charge On The ElectronskruzerNo ratings yet
- Planet CompositionDocument1 pagePlanet CompositionskruzerNo ratings yet
- Si UnitsDocument12 pagesSi UnitsskruzerNo ratings yet
- Organizing The ElementsDocument1 pageOrganizing The ElementsskruzerNo ratings yet
- Periodic Table With Masses and NumbersDocument1 pagePeriodic Table With Masses and NumbersskruzerNo ratings yet
- Calculate The Molecular Mass of CompoundsDocument1 pageCalculate The Molecular Mass of CompoundsskruzerNo ratings yet
- Planetary Density, Size, and AtmosphereDocument1 pagePlanetary Density, Size, and AtmosphereskruzerNo ratings yet
- Melting Points of The Elements CDocument1 pageMelting Points of The Elements CskruzerNo ratings yet
- Crystal Structure of Metals Lattice Structure 0Document1 pageCrystal Structure of Metals Lattice Structure 0skruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Luminescence Atomic StructureDocument1 pageLuminescence Atomic StructureskruzerNo ratings yet
- Energy Levels HydrogenDocument1 pageEnergy Levels HydrogenskruzerNo ratings yet
- Geiger and Marsden's ApparatusDocument1 pageGeiger and Marsden's ApparatusskruzerNo ratings yet
- Investigating The Electron 1Document1 pageInvestigating The Electron 1skruzerNo ratings yet
- Crystal Structure of Metals Efficient PackingDocument1 pageCrystal Structure of Metals Efficient PackingskruzerNo ratings yet
- Cathode Ray OscilloscopeDocument1 pageCathode Ray OscilloscopeskruzerNo ratings yet
- Determination of Avogadro's ConstantDocument1 pageDetermination of Avogadro's ConstantskruzerNo ratings yet
- Investigating The Electron 2Document1 pageInvestigating The Electron 2skruzerNo ratings yet
- Boiling Points of The Elements CDocument1 pageBoiling Points of The Elements CskruzerNo ratings yet
- Atomic Volumes of The ElementsDocument1 pageAtomic Volumes of The ElementsskruzerNo ratings yet
- Atomic StructureDocument1 pageAtomic StructureskruzerNo ratings yet
- Atomic Emission Spectrum HydrogenDocument1 pageAtomic Emission Spectrum HydrogenskruzerNo ratings yet
- Atomic MassDocument1 pageAtomic MassskruzerNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)