Professional Documents
Culture Documents
ld50 Lab Report
Uploaded by
api-267601782Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ld50 Lab Report
Uploaded by
api-267601782Copyright:
Available Formats
LD50 Lab Report
Luke Johnson
Mrs. Norris
AP Environmental Science
11 October, 2014
Collaborators:
Hannah Whitt, James Hamil, Megan Redfern
Introduction:
It is suspected that salt (NaCl sodium chloride) applied to highways for deicing may affect the
growth of vegetation along the roadside and aquatic plants in nearby streams. Therefore, a dose-
response experiment must be conducted to determine how radish seeds will respond to various
concentrations of salt. Vegetation supports entire ecosystems by providing a source of food for animals.
Vegetation also prevents erosion along roads. If salt is affects aquatic and land vegetation, then
measures must be taken to prevent the use of salt to remove ice on roads.
Problem:
Do higher concentrations of salt solutions in water contribute to fewer germinating radish
seeds?
Hypothesis:
The higher concentration of salt solution in water, the fewer radish seeds will germinate. 100%
of seeds will germinate for the control group, 100% of seeds will germinate from the .75 g NaCl/L H2O,
90% of seeds will germinate from 1.5 g NaCl/L H2O, 80% will germinate from the 3 g NaCl/L H2O, 60%
will germinate from the 6 g NaCl/L H2O, 40% will germinate from the 12 g NaCl/L H2O. The seeds that
are able to germinate with higher salt solutions will experience depressed growth.
Parts of the Experiment:
Control Group: 10 mL of distilled water (Container 6)
Experimental Group: radish seeds that are exposed to various concentrations of salt
Independent Variable: concentration of salt solutions in g NaCl/L H20
Dependent Variable: Percentage of seeds that germinated and radicle growth
Controlled variable: Room temperature, environment, amount of water each seed
receives, amount of light each plant recieves
Materials: (Lab Protocol #1)
6 test tubes (size 20 X 150 mm)
Tape (for labeling test tubes)
Test tube rack
10 mL graduated cylinder or pipette
Distilled water
concentrated salt solution = 12.0 grams table salt (sodium chloride)/1 liter distilled
water
Materials: (Lab Protocol #2)
6 test tubes with serial dilutions of salt solutions (from part 1)
6-90 mm petri dishes with lids
3 pieces of unbleached paper towel
Pencil
Scissors
10 mL graduated cylinder or syringe
Tape and permanent marker
60 lettuce seeds (Radish seeds, 10/petri dish)
Tweezers
Plastic bag
Materials: (Lab Protocol #3)
Metric ruler
Calculator
2 sheets graph paper (75 square X 100 squares)
Methods: (Lab Protocol #1)
1. Set up 6 test tubes in a test tube rack and label the tubes with the following salt concentrations:
12.0 g/L, 6.0 g/L, 3.0 g/L, 1.5 g/L, 0.75 g/L and Control. See Table 1. Salt Solutions.
2. Add 10 mL of distilled water to test tubes #2-6.
3. Measure 20 mL of the concentrated solution (12.0 g/L) and pour into test tube #1.
4. Transfer 10 mL of salt solution from test tube # 1 to test tube #2.
5. Gently swirl test tube #2 to mix the salt solution.
6. Repeat steps 4 and 5 for test tubes #3-5 measuring 10 mL each time. DO NOT add any salt
solution to test tube #6.
7. Measure 10 mL of distilled water into test tube #6 to serve as the control. The control will
indicate whether or not your seeds are viable (capable of growing or developing). NOTE: The
total remaining solution in each test tube is 10 mL, except for test tube #5 will have 20 mL.
8. Unless you will be using the solutions right away, cover them tightly with plastic wrap to prevent
water loss through evaporation.
Methods: (Lab Protocol #2)
1. Obtain six petri dishes. Label each dish according to the concentration of salt solution to be
tested. See Table 1. Salt Solutions Concentrations.
2. Fold a half of sheet of paper towel or coffee filter into quarters. Cut it out so that it fits into the
bottom of the petri dish.
3. Measure 6 mL of salt solution and pour onto the paper towel in the appropriate petri dish. In
the control dish, add 5 mL of distilled water. The purpose of a control is to identify how well the
seeds will grow without any salt. NOTE: If using the same graduated cylinder, start with the
lowest salt solution (distilled water) to the highest salt solution so that the graduated cylinder is
not contaminated with a higher salt solution.
4. Add 10 lettuce seeds to each petri dish. Space the seeds out evenly on the paper towel so that
they do not touch each other or the sides of the dish.
5. Place the dishes in a plastic bag and seal it to retain moisture. Label your groups name on the
outside of the bag.
6. Incubate the seeds in a dark place at a constant temperature (preferably 24.5 degrees Celsius)
for 4-5 days.
7. Inspect radish seeds during incubation period. Record any observations. If the paper seems dry,
add a 1 or 2 more millimeters of the appropriate salt solution or distilled water (control).
Methods: (Lab Protocol #3)
1. Remove the lid of the control dish. Count the number of seeds that germinated (sprouted).
Calculate the percentage of seeds that germinated and record in Table 2. Radish Seed Results.
Note: If fewer than 80% of the seeds in the control sample germinated, this indicates a
problem with the experiment, e.g., bad seeds, poor incubation conditions, etc. The results
discarded or the test rerun.
2. To measure the length of the radicle (embryonic root), carefully remove the germinating radish
seed from the paper towel in one piece. The radicle may be growing into the layers of towel and
can break if you pull too hard.
3. Measure the length of the radicle for each of the germinating radish seeds to the nearest
millimeter (mm). Look carefully at each sprout to make sure you are measuring just the root, not
the shoot as well. Record data in Table 2. Radish Seed Results.
4. Repeat steps 1-3 for each petri dish.
5. For each treatment, calculate the mean (arithmetic average) radicle length for each salt
solution. Add the total radicle lengths for each salt solution and divide by the total number of
seeds that germinated. DO NOT INCLUDE data from seeds that did not germinate. Record data
in column labeled, Mean Radicle Length (mm) in Table 2. Radish Seed Results.
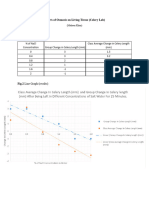
6. Make a line graph from the data collected to show a dose-response curve. The horizontal axis
should be for the independent variable, dose (concentration of salt solutions). The vertical axis
should be for the dependent variable, response (mean radicle length)/ Draw and label the axes.
In your group, you will need to come to some agreement about scales for these by looking at
your data. Remember to give your graph a title.
7. To help you answer the following question: Did the radicle length increase or decrease in
length as compared to the control? subtract the mean radicle length of ach treatment (T) from
the mean radicle length of the control (C). Record your answers in the column, Difference in
Radicle Length on Table 2. Difference in Radicle Length = C (control) T (treatment mean
radicle length)
8. Make a line graph to show the percentage of seeds that germinated for each salt solution.
Complete your lab report. See Student Handout 2: Laboratory Report Outline.
Photos:
Data:
Group 6 Data
Treatments:
Concentration of
Salt Solution
(mg/L)
% Seeds
Germinated
1
mm
2
mm
3
mm
4
mm
5
mm
6
mm
7
mm
8
mm
9
mm
10
mm
T= Mean
Radicle
Length
(mm)
Difference
in Radicle
Length:
C- T
C = Control 100% 75 50 70 60 80 70 85 60 85 90 72.5 0
.75 100% 100 43 62 73 34 57 26 70 60 36 56.1 16.4
1.5 100% 95 62 116 70 80 50 75 100 60 85 79.3 -6.8
3.0 100% 45 30 95 40 75 30 45 30 35 60 48.5 24
6.0 100% 66 35 56 41 73 43 49 40 75 30 50.8 21.7
12.0 100% 16 15 20 5 20 0 0 0 0 0 7.6 64.9
Group Number
Mean Radicle Length (mm) 1 2 3 4 5 6 7 8
Control 81.5 78 89.7 121.8 35.3 72.5 41
19.5
12.0 34 26 29.5 35.0 49 7.6 42.5
.7
6.0 78.5 40 55 99.7 0 50.8 45
12
3.0 135.5 72 68.5 105.0 52.1 48.5 71
8.5
1.5 82.5 82 100.7 110.6 47.1 79.3 45.5
45.5
.75 97.8 100 118.6 83.34 50.7 56.1 22.5
15.5
Group 1 2 3 4 5 6 7
8
% Seeds Germinated
Control 100% 100% 100% 100% 100% 100% 100%
100%
12.0 80% 40% 40% 60% 70% 50% 90%
40%
6.0 100% 90% 80% 100% 0% 100% 100%
90%
3.0 100% 80% 80% 90% 80% 100% 100%
80%
1.5 90% 100% 100% 100% 70% 100% 90%
100%
.75 100% 100% 100% 90% 100% 100% 80%
90%
Difference in Radicle Length
(mm)
Length of Control 87.5 78
121.8 0
0
12.0 47.7 52 60.2 -86.8 -13.7 -64.9 -18.5
18.8
6.0 3 38 34.7 -22.1 35.3 -21.7 4.5
7.5
3.0 -5.4 6 21.2 -16.8 -16.8 -24 30
11
1.5 -1 -4 -11 -11.2 -11.8 6.8 4
26
.75 -16.5 -22 -28.9
-
38.47
-15.4 -16.4 1.5
4
Mean Radical Length (mm)
(Figure 1)- Each line represents a separate group
% Seeds Germinated
(Figure 2)- Each line represents a different group
0
20
40
60
80
100
120
140
160
Control 12 6 3 1.5 0.75
1
2
3
4
5
6
7
8
0%
20%
40%
60%
80%
100%
120%
Control 12 6 3 1.5 0.75
1
2
3
4
5
6
7
8
Difference in Radical Length
(Figure 3)- Each line represents a different group
Conclusion:
The control group had 100% germination for 100% of the groups, meaning the lab was
performed correctly and most likely did not have bad seeds, or poor incubation conditions. Some groups
did experience heightened growth of radish seed roots as compared to the control in salt solutions such
as in groups three and five where the .75 g NaCl/L H20 had longer roots than the control. As the salt
solutions became increasingly concentrated with salt, the radicle length did decrease as compared to
the control a majority of the time. The only discrepancy may be the 0% of seeds germinate in figure 2 for
group 5s salt concentration of 6 g NaCl/L H2O because not a single other group had 0% germinated
even for higher concentrations of salt. It appears that lower concentrations of salt water do not greatly
-100
-50
0
50
100
150
Length of
Control
12 6 3 1.5 0.75
1
2
3
4
5
6
7
8
impact radish germination, but radicle growth is somewhat effected, though higher concentrations have
a very clear negative impact and should not be used to deice roads.
Work Cited
"LD50 Lab Directions.pdf - Google Drive." LD50 Lab Directions.pdf - Google Drive. N.p., n.d. Web. 12 Oct.
2014.
You might also like
- ld50 Lab ReportDocument9 pagesld50 Lab Reportapi-363325485No ratings yet
- ld50 LabDocument9 pagesld50 Labapi-286182524No ratings yet
- ld50 Lab 2015Document5 pagesld50 Lab 2015api-279856678No ratings yet
- ld50 LabDocument5 pagesld50 Labapi-408976248No ratings yet
- UntitledDocument12 pagesUntitledjNo ratings yet
- Writing Sample 1Document7 pagesWriting Sample 1api-582848179No ratings yet
- ld50 LabDocument6 pagesld50 Labapi-277689164No ratings yet
- Exemplary Internal AssesmentDocument12 pagesExemplary Internal AssesmentSeán ByrneNo ratings yet
- 2 H O 2 H O + O: General DirectionsDocument5 pages2 H O 2 H O + O: General Directionssplink82No ratings yet
- The Effect of Salinity On The Growth of Phaseolus VulgarisDocument11 pagesThe Effect of Salinity On The Growth of Phaseolus VulgarisPhilip KaulNo ratings yet
- Sick Plants ExperimentDocument5 pagesSick Plants ExperimentThomas RichardNo ratings yet
- Keloid Cell Line SubcultureDocument4 pagesKeloid Cell Line SubcultureNguyen Dac Hanh HieuNo ratings yet
- The Effect of Ethanol On Membrane PermeabIlIty in BeetrootDocument4 pagesThe Effect of Ethanol On Membrane PermeabIlIty in BeetrootHowsit Slouching60% (5)
- AQA A-Level Biology Required Practical Methods & Notes: Name: Class: Biology TeacherDocument43 pagesAQA A-Level Biology Required Practical Methods & Notes: Name: Class: Biology TeacherJoJoNo ratings yet
- 261 Labs - U1 - Osmolarity - Lab ReportDocument12 pages261 Labs - U1 - Osmolarity - Lab ReportSummerNo ratings yet
- Conducting Reference Toxicity Tests With Lettuce SeedsDocument5 pagesConducting Reference Toxicity Tests With Lettuce Seedsapi-32133818No ratings yet
- The Effect of Detergent Concentration On Plant Growth: Erin Annunziato Ms. PietrangeloDocument19 pagesThe Effect of Detergent Concentration On Plant Growth: Erin Annunziato Ms. PietrangeloAngela_2410No ratings yet
- Osmosis BeetrootDocument5 pagesOsmosis BeetrootNicolas Duquenne100% (1)
- Método PrataDocument6 pagesMétodo PrataCorinne MartinNo ratings yet
- Ab65475 Quick Cell Proliferation Assay Kit II Protocol v2 (Website)Document12 pagesAb65475 Quick Cell Proliferation Assay Kit II Protocol v2 (Website)Venkatesh GaviniNo ratings yet
- Bio411 Lab Report 3Document12 pagesBio411 Lab Report 3Nur Aqillah100% (1)
- Effect of Different Levels of Sugar in Pulsing Treatment On Post Harvest Quality of Gladiolus Cv. 'American Beauty'Document5 pagesEffect of Different Levels of Sugar in Pulsing Treatment On Post Harvest Quality of Gladiolus Cv. 'American Beauty'Dr Parag B JadhavNo ratings yet
- Osmosis BC BiologicalMembranesPrac (Salt, Beetroot)Document5 pagesOsmosis BC BiologicalMembranesPrac (Salt, Beetroot)Niki LiNo ratings yet
- Osmolarity LabDocument2 pagesOsmolarity LabPhi KhanhNo ratings yet
- SA VolformativeDocument5 pagesSA VolformativeSamantha WidjajaNo ratings yet
- Useful Numbers Y14472 Useful NmbrsDocument1 pageUseful Numbers Y14472 Useful NmbrsnanopearlNo ratings yet
- Diffusion and Osmosis Lab Essential Question: Why Is It Important For Intravenous Fluids Given in A Hospital To HaveDocument6 pagesDiffusion and Osmosis Lab Essential Question: Why Is It Important For Intravenous Fluids Given in A Hospital To HavecrystalNo ratings yet
- Apes Salinization LabDocument4 pagesApes Salinization LabYujin LeeNo ratings yet
- AICE Biology Osmosis Lab: Plasmolysis in Plant Tissue: BackgroundDocument3 pagesAICE Biology Osmosis Lab: Plasmolysis in Plant Tissue: BackgroundWiji NingNo ratings yet
- Beetroot PracDocument7 pagesBeetroot Pracapi-288543574No ratings yet
- 6.02 Experiment 6 Root Pressure PreparationDocument1 page6.02 Experiment 6 Root Pressure PreparationNakka HemanthNo ratings yet
- Analytical Biochemistry PDFDocument40 pagesAnalytical Biochemistry PDFharpreet0% (1)
- Post Harvest HandlingDocument61 pagesPost Harvest HandlingJagadish HortiNo ratings yet
- CM-1 24957-00 CorrosionManagement PDFDocument28 pagesCM-1 24957-00 CorrosionManagement PDFSamirNo ratings yet
- DOC316.53.01145 Ed7Document6 pagesDOC316.53.01145 Ed7Noel LamNo ratings yet
- Effects of Osmosis On Living Tissue (Celery Lab)Document5 pagesEffects of Osmosis On Living Tissue (Celery Lab)animeanimism12No ratings yet
- Biology Lab Report - Osmosis-: The Effect of Various Concentrations of Nacl Salt Solutions On The Length of Potato StripsDocument13 pagesBiology Lab Report - Osmosis-: The Effect of Various Concentrations of Nacl Salt Solutions On The Length of Potato StripsNoor Amrun0% (2)
- SpeechDocument14 pagesSpeechCarlosNo ratings yet
- LettuceseedlabDocument4 pagesLettuceseedlabapi-357486814No ratings yet
- University Committee On Animal Care and Supply (UCACS) Standard Operating Procedure (SOP)Document36 pagesUniversity Committee On Animal Care and Supply (UCACS) Standard Operating Procedure (SOP)Eusebia Josephine CuaresmaNo ratings yet
- Quality of Tomato Seedlings Grown in Modified Floating SystemDocument6 pagesQuality of Tomato Seedlings Grown in Modified Floating SystemPremier PublishersNo ratings yet
- USP Sieve SizeDocument5 pagesUSP Sieve Sizejayvee franciscoNo ratings yet
- Salinity and It's Effect On The Germination Rates of Radishes - Ranier KnowlesDocument8 pagesSalinity and It's Effect On The Germination Rates of Radishes - Ranier KnowlesRanierNo ratings yet
- Detergent LabDocument8 pagesDetergent LabInsen BacharNo ratings yet
- Experimental Design Diagram and Independent VariablesDocument4 pagesExperimental Design Diagram and Independent Variablesalain presillasNo ratings yet
- CalciumassayprotocolDocument9 pagesCalciumassayprotocolapi-249800205No ratings yet
- Edexcel A-Level Biology Experimental Design Marks Scheme (1) (Full Permission)Document16 pagesEdexcel A-Level Biology Experimental Design Marks Scheme (1) (Full Permission)FardeenKhanNo ratings yet
- CAPE Biology Labs - OsmosisDocument9 pagesCAPE Biology Labs - OsmosisAshleigh SmithNo ratings yet
- Determining The Water Potential of Potato Tuber Cells by The Weighting MethodDocument2 pagesDetermining The Water Potential of Potato Tuber Cells by The Weighting MethodDuaa Saqri RamahiNo ratings yet
- Effect Varying Dosage of Gamma Irradiation On The Growth of Zea MaysDocument14 pagesEffect Varying Dosage of Gamma Irradiation On The Growth of Zea MaysShannen SenaNo ratings yet
- Synchronous Cultures By: of Bacillus Subtilis Obtained Filtration With Glass Fiber FiltersDocument5 pagesSynchronous Cultures By: of Bacillus Subtilis Obtained Filtration With Glass Fiber FiltersPrashant PutariyaNo ratings yet
- Osmosis InvestigationDocument9 pagesOsmosis InvestigationvntexvnNo ratings yet
- Classification and Ordination Methods As A Tool For Analyzing of Plant CommunitiesDocument34 pagesClassification and Ordination Methods As A Tool For Analyzing of Plant CommunitiespcorgoNo ratings yet
- CRE ManualDocument51 pagesCRE ManualAli NawazNo ratings yet
- Green Lab 2Document4 pagesGreen Lab 2TEN CHEANGNo ratings yet
- Osmosis Demonstration LabDocument3 pagesOsmosis Demonstration LabMichael KrásaNo ratings yet
- Chemical Analysis of Non-antimicrobial Veterinary Drug Residues in FoodFrom EverandChemical Analysis of Non-antimicrobial Veterinary Drug Residues in FoodJack F. KayNo ratings yet
- NPP Lab ReportDocument7 pagesNPP Lab Reportapi-267601782No ratings yet
- Leaf Litter Lab ReportDocument6 pagesLeaf Litter Lab Reportapi-2676017820% (1)
- Something Fishy Lab ReportDocument6 pagesSomething Fishy Lab Reportapi-267601782No ratings yet
- Ocean Acidification Lab ReportDocument4 pagesOcean Acidification Lab Reportapi-267601782No ratings yet
- 191 - 197 - Detection of Transaction Fraud Using Deep LearningDocument28 pages191 - 197 - Detection of Transaction Fraud Using Deep LearningADRINEEL SAHANo ratings yet
- PHYSICS Lab Manual - 2023-24Document30 pagesPHYSICS Lab Manual - 2023-24Vinushree Santhoshkumar100% (4)
- Silvaco ATHENA Description 1 PDFDocument18 pagesSilvaco ATHENA Description 1 PDFRahul JaiswalNo ratings yet
- Asco Series 042 Gas Shutoff CatalogDocument4 pagesAsco Series 042 Gas Shutoff CatalogRoqueNetNo ratings yet
- This Study Resource Was: EvaluateDocument2 pagesThis Study Resource Was: EvaluateMary angel PerjesNo ratings yet
- 125 Tractor: (Specifications and Design Subject To Change Without Notice)Document5 pages125 Tractor: (Specifications and Design Subject To Change Without Notice)Gary LarsonNo ratings yet
- Abb Sas GeneralDocument43 pagesAbb Sas Generalsabill arasyidNo ratings yet
- (FreeCourseWeb - Com) 1493997599Document386 pages(FreeCourseWeb - Com) 1493997599MuruganandamGanesanNo ratings yet
- Metric Ton Is 1000 KGDocument5 pagesMetric Ton Is 1000 KGmcpayodNo ratings yet
- LEC# 15. Vapor Compression, Air ConditioningDocument31 pagesLEC# 15. Vapor Compression, Air ConditioningAhmer KhanNo ratings yet
- Science July Assignment Grade 8Document3 pagesScience July Assignment Grade 8G PNo ratings yet
- MDM Heiana Nadia Hamzah: Prepared byDocument50 pagesMDM Heiana Nadia Hamzah: Prepared bySyarfa FurzanneNo ratings yet
- Cross-Cultural Validation of The Scales For Outcomes in Parkinson's Disease-Psychosocial Questionnaire (SCOPA-PS) in Four Latin American CountriesDocument7 pagesCross-Cultural Validation of The Scales For Outcomes in Parkinson's Disease-Psychosocial Questionnaire (SCOPA-PS) in Four Latin American Countriesfozia hayyatNo ratings yet
- Calibar Plate3Document10 pagesCalibar Plate3Gerald FernandezNo ratings yet
- Visit Braindump2go and Download Full Version 350-801 Exam DumpsDocument4 pagesVisit Braindump2go and Download Full Version 350-801 Exam DumpsArsic AleksandarNo ratings yet
- Correlated Optical Convolutional Neural Network With "Quantum Speedup"Document27 pagesCorrelated Optical Convolutional Neural Network With "Quantum Speedup"jaccneeNo ratings yet
- TUC5+ Modbus ID Details PDFDocument10 pagesTUC5+ Modbus ID Details PDFvijikeshNo ratings yet
- Ch24 TestbankDocument40 pagesCh24 TestbankIannah Malvar100% (1)
- Structural Design and Optimization - Part IIDocument448 pagesStructural Design and Optimization - Part IIFranco Bontempi100% (1)
- Astar - 23b.trace - XZ Bimodal Next - Line Next - Line Next - Line Next - Line Drrip 1coreDocument4 pagesAstar - 23b.trace - XZ Bimodal Next - Line Next - Line Next - Line Next - Line Drrip 1corevaibhav sonewaneNo ratings yet
- QuesTeksFerriumC61C64andC6 PDFDocument23 pagesQuesTeksFerriumC61C64andC6 PDFEmily MillerNo ratings yet
- Tefnol 1Document11 pagesTefnol 1Moustapha Salem MansourNo ratings yet
- New EM Quiz13Document4 pagesNew EM Quiz13Singh KaranNo ratings yet
- Activity 2 Resultant Vector by Graphical MethodDocument2 pagesActivity 2 Resultant Vector by Graphical MethodRick Ignacio0% (1)
- Supercritical CO2: Properties and Technological Applications - A ReviewDocument38 pagesSupercritical CO2: Properties and Technological Applications - A ReviewXuân ĐứcNo ratings yet
- Math10 - Q1 - Mod3 - DeterminingArithmetic - V3 1Document28 pagesMath10 - Q1 - Mod3 - DeterminingArithmetic - V3 1Cristina Amantiad100% (5)
- Decision Analysis: Learning ObjectivesDocument30 pagesDecision Analysis: Learning ObjectivesMorgan AfrizalNo ratings yet
- Pso NS2Document6 pagesPso NS2sankarideviNo ratings yet
- Deflection of BeamsDocument109 pagesDeflection of BeamsNadir Khattak Jr.100% (1)