Professional Documents
Culture Documents
Polypeptides Polypeptides
Uploaded by
dreamboy87Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Polypeptides Polypeptides
Uploaded by
dreamboy87Copyright:
Available Formats
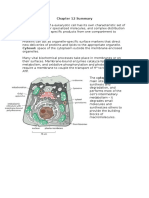
ATP binds to the A subunits and it is then hydrolyzed to power the alternation, but the exact
process by which this happens is not known. The four domains can be present in four
separate polypeptides, which occur mostly in bacteria, or present in one or two multidomain polypeptides.[1] When the polypeptides are one domain, they are can be referred to as a
full domain, and when they are two multi-domains they can be referred to as a half domain.[3] The
T domains are each built of typically 10 membrane spanning alpha helices, through which the
transported substance can cross through the plasma membrane. Also, the structure of the T
domains determines the specificity of each ABC protein. In the inward facing conformation, the
binding site on the A domain is open directly to the surrounding aqueous solutions. This allows
hydrophilic molecules to enter the binding site directly from the inner leaflet of the phospholipid
bilayer. In addition, a gap in the protein is accessible directly from the hydrophobic core of the
inner leaflet of the membrane bilayer.
You might also like
- B&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BDocument4 pagesB&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BbahlasaNo ratings yet
- ClaseDocument7 pagesClasef.tellelopezNo ratings yet
- Cell Transport Review ANSWERSDocument7 pagesCell Transport Review ANSWERSAnonymous pUJNdQNo ratings yet
- Solution BiotechDocument54 pagesSolution Biotechkavita sahaiNo ratings yet
- Booth Clarke Plegamiento 2010Document2 pagesBooth Clarke Plegamiento 2010Rayo McQueenNo ratings yet
- Membrane Structure and Function: Lecture OutlineDocument11 pagesMembrane Structure and Function: Lecture Outlinehaha_le12No ratings yet
- BIOC 2061 Tutorial 3Document4 pagesBIOC 2061 Tutorial 3Kavita MaharajNo ratings yet
- Worksheet Unit 6 - MembranesDocument2 pagesWorksheet Unit 6 - MembranesShooting StarNo ratings yet
- 1 Strcutural Bio NotesDocument15 pages1 Strcutural Bio NotesAngel TaylorNo ratings yet
- Molecular Cell Biology Lodish 7th Edition Solutions ManualDocument8 pagesMolecular Cell Biology Lodish 7th Edition Solutions ManualKennethHendersonyecg100% (40)
- BioChe Ch. 9Document3 pagesBioChe Ch. 9JoyceLingNo ratings yet
- Chapter 3Document24 pagesChapter 3Dawlat Slama0% (1)
- Research Paper On Plasma MembraneDocument4 pagesResearch Paper On Plasma Membranevvgnzdbkf100% (1)
- Exam 1 Lectures 4 & 5 Q'sDocument6 pagesExam 1 Lectures 4 & 5 Q'sBlaNo ratings yet
- BioPhysics New MCQDocument95 pagesBioPhysics New MCQAchraf RabadiNo ratings yet
- Mechanism of The Eukaryotic Chaperonin: Protein Folding in The Chamber of SecretsDocument7 pagesMechanism of The Eukaryotic Chaperonin: Protein Folding in The Chamber of SecretsDeependra Kumar BanNo ratings yet
- Super Secondary StructureDocument4 pagesSuper Secondary Structureprism1702No ratings yet
- Solution Manual For Molecular Cell Biology 8th Edition Harvey Lodish Arnold Berk Chris A Kaiser Monty Krieger Anthony Bretscher Hidde Ploegh Angelika Amon Kelsey C Martin 33Document5 pagesSolution Manual For Molecular Cell Biology 8th Edition Harvey Lodish Arnold Berk Chris A Kaiser Monty Krieger Anthony Bretscher Hidde Ploegh Angelika Amon Kelsey C Martin 33PhilipWoodpsen100% (38)
- H - Atpases in The Plasma Membrane: Physiology and Molecular BiologyDocument22 pagesH - Atpases in The Plasma Membrane: Physiology and Molecular BiologyPhan Quoc TamNo ratings yet
- Plasma MembraneDocument18 pagesPlasma MembraneJemuel Bucud LagartoNo ratings yet
- CATALASEDocument9 pagesCATALASEJed Dumadag Dano100% (3)
- Bacteria From PrescottDocument19 pagesBacteria From PrescottYekitaSNo ratings yet
- CHEM 151 (Chapter 4)Document4 pagesCHEM 151 (Chapter 4)Chantel Acevero100% (1)
- IB HL Biology Notes Cell MembranesDocument12 pagesIB HL Biology Notes Cell MembranesayushfmNo ratings yet
- Protein Structure: Building BlockDocument7 pagesProtein Structure: Building BlockMadigani PrasannaNo ratings yet
- Chapter 7 Study GuideDocument9 pagesChapter 7 Study GuideCesar MontenegroNo ratings yet
- Biological Cell MembranesDocument44 pagesBiological Cell MembranesMohammed ShaikNo ratings yet
- Chap 11Document14 pagesChap 11Tai DonovanNo ratings yet
- Ban ̃o ́-Polo-2012Document8 pagesBan ̃o ́-Polo-2012sencer55No ratings yet
- Proteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDocument12 pagesProteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDanielle GuerraNo ratings yet
- Markscheme HL Paper2Document56 pagesMarkscheme HL Paper2George KoukouvesNo ratings yet
- BCH 203 & 202Document28 pagesBCH 203 & 202metasynthronos748No ratings yet
- BT102 Microbiology Current Paper Solved Question Mid Term December DownloadDocument9 pagesBT102 Microbiology Current Paper Solved Question Mid Term December Downloadbc230422430skhNo ratings yet
- Marine Guillot Et Al - Effects of Structural Modification On Gene Transfection and Self-Assembling Properties of Amphiphilic DendrimersDocument4 pagesMarine Guillot Et Al - Effects of Structural Modification On Gene Transfection and Self-Assembling Properties of Amphiphilic DendrimersKorezmNo ratings yet
- 008 - The Cell Surface MembraneDocument2 pages008 - The Cell Surface MembraneSanah KhanNo ratings yet
- Proteolysis and Chaperones The Destruction Reco - 1998 - Current Opinion in MicDocument6 pagesProteolysis and Chaperones The Destruction Reco - 1998 - Current Opinion in MicJuan Pablo Vizcaya GuependoNo ratings yet
- Chapter 12 SummaryDocument7 pagesChapter 12 SummaryCharlotteNo ratings yet
- Plasma Membrane in DetailsDocument24 pagesPlasma Membrane in DetailsSyeda Kaneez Mohammadi MohammadiNo ratings yet
- Mark Schemes Edexcel Synoptic EssaysDocument9 pagesMark Schemes Edexcel Synoptic Essaysapi-3740691100% (1)
- Lipids, Membranes and Transport OutlineDocument25 pagesLipids, Membranes and Transport Outlinefalcons22No ratings yet
- Protein Structure PDFDocument6 pagesProtein Structure PDFmradu1No ratings yet
- A Membrane Is A Lipid Bilayer With Proteins Embedded in ItDocument3 pagesA Membrane Is A Lipid Bilayer With Proteins Embedded in ItDahniar Nur AisyahNo ratings yet
- LectureNotes112 5Document3 pagesLectureNotes112 5S Young LeeNo ratings yet
- Protein Synthesis: By: Jerome S. Montano, RMTDocument24 pagesProtein Synthesis: By: Jerome S. Montano, RMTAhuNo ratings yet
- Cellular Respiration and PhotosynthesisDocument2 pagesCellular Respiration and PhotosynthesisDonna O.No ratings yet
- Problems Fluid MosaickeyDocument7 pagesProblems Fluid MosaickeyBatrisyia AdNo ratings yet
- Plasma Membrane Research PapersDocument6 pagesPlasma Membrane Research Papersaflbasnka100% (1)
- Biochemistry ProblemDocument5 pagesBiochemistry ProblemJnanendra SenguptaNo ratings yet
- Cell Membranes and SignalingDocument7 pagesCell Membranes and Signalingbasmala6545No ratings yet
- Biochemistry Ch. 2 ProteinsDocument7 pagesBiochemistry Ch. 2 ProteinsBryantNo ratings yet
- Lipid Movement BiochemDocument9 pagesLipid Movement BiochemCrowNo ratings yet
- 5 - Biochemistry - 3rd Year Chem - Biological Membranes and Transports - Chapter-5 - Part 3Document27 pages5 - Biochemistry - 3rd Year Chem - Biological Membranes and Transports - Chapter-5 - Part 3MD. SAJID GHUFRANNo ratings yet
- Unit 4Document86 pagesUnit 4vanitapanda14959No ratings yet
- BCH MCQs1Document304 pagesBCH MCQs1moxdegr8100% (1)
- IB Biology Revision SpreadsheetDocument124 pagesIB Biology Revision SpreadsheetTanika SharmaNo ratings yet
- Study Exercise Biology 10-12 97.03Document7 pagesStudy Exercise Biology 10-12 97.03Fabiola Vania FeliciaNo ratings yet
- Ch. 5 Pre-ReadingDocument7 pagesCh. 5 Pre-ReadingDJ ISAACS50% (2)
- 16-02-21 Class PDFDocument13 pages16-02-21 Class PDFDebopam RayNo ratings yet
- Zoology Lecture 4Document18 pagesZoology Lecture 4Stephen OcheaNo ratings yet
- Energy Conservation at Macro LevelDocument53 pagesEnergy Conservation at Macro Leveldreamboy87No ratings yet
- 3.AH PerformanceDocument20 pages3.AH Performancedreamboy87No ratings yet
- Chapter - VII Infrastructure Water SupplyDocument25 pagesChapter - VII Infrastructure Water Supplydreamboy87No ratings yet
- Water Treatment Plant Process Flow & Description: by Chemical Department Vendor: Doshion Veolia Water SolutionsDocument8 pagesWater Treatment Plant Process Flow & Description: by Chemical Department Vendor: Doshion Veolia Water Solutionsdreamboy87No ratings yet
- Methods and Effects of Manpower TrainingDocument17 pagesMethods and Effects of Manpower Trainingdreamboy87No ratings yet
- 4.mill PerfDocument30 pages4.mill PerfRAJESH KUMARNo ratings yet
- 8 SP Oil, Cap RednDocument8 pages8 SP Oil, Cap Redndreamboy87No ratings yet
- SelfDeclarationForm TN 620190926329Document1 pageSelfDeclarationForm TN 620190926329dreamboy87No ratings yet
- Tur ProtnsDocument2 pagesTur Protnsdreamboy87No ratings yet
- Formula Study GuideDocument8 pagesFormula Study Guidemusharat_shafiqueNo ratings yet
- Train Schedule Booklet SettingDocument24 pagesTrain Schedule Booklet Settingdreamboy87100% (1)
- Turbine Oil System: P & I Check List.: Required ConditionsDocument4 pagesTurbine Oil System: P & I Check List.: Required Conditionsdreamboy87100% (1)
- Status Basic EngineeringDocument19 pagesStatus Basic Engineeringdreamboy87No ratings yet
- Inspection Checklist AugustDocument3 pagesInspection Checklist Augustdreamboy87No ratings yet
- SHE Manual For Construction Contractors-EditDocument79 pagesSHE Manual For Construction Contractors-Editdreamboy87100% (1)
- Work at Height InventoryDocument1 pageWork at Height Inventorydreamboy87No ratings yet
- PTW 1Document8 pagesPTW 1dreamboy87No ratings yet
- Emergency Response Plan For Construction Site-EditDocument11 pagesEmergency Response Plan For Construction Site-Editdreamboy87100% (4)
- Commissioning ScheduleDocument1 pageCommissioning Scheduledreamboy87No ratings yet
- Ref: Work Permit No:: Honeywell International ME-HBS - Dubai Site-JBR-CCTV Upgrade Special Work PermitDocument6 pagesRef: Work Permit No:: Honeywell International ME-HBS - Dubai Site-JBR-CCTV Upgrade Special Work Permitdreamboy87No ratings yet
- Construction Safety Manual-EditDocument65 pagesConstruction Safety Manual-Editdreamboy87100% (2)
- Concept Note For HSD Storage / HandlingDocument3 pagesConcept Note For HSD Storage / Handlingdreamboy87No ratings yet
- Construction Contractor Safety Guidelines-EditDocument5 pagesConstruction Contractor Safety Guidelines-Editdreamboy87No ratings yet
- Silt Content TestDocument3 pagesSilt Content Testdreamboy87100% (2)
- A 2 Z India 01-2019Document24 pagesA 2 Z India 01-2019dreamboy87No ratings yet
- J. Subbiah,: Thermal Power Station-Ii Neyveli Lignite Corporation Limited NeyveliDocument55 pagesJ. Subbiah,: Thermal Power Station-Ii Neyveli Lignite Corporation Limited Neyvelidreamboy87No ratings yet
- Checklist For RMCDocument23 pagesChecklist For RMCAjinNo ratings yet
- 3393 - Supervision of Concrete Construction Volume 2Document271 pages3393 - Supervision of Concrete Construction Volume 2Oum ChhayNoy100% (11)
- NLC Corrigendum For Ball ClayDocument3 pagesNLC Corrigendum For Ball Claydreamboy87No ratings yet
- Ballclay Sale 201601Document2 pagesBallclay Sale 201601dreamboy87No ratings yet