Professional Documents
Culture Documents
Mathcad 05 - 6 Evapor#F7A08
Uploaded by
cymyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mathcad 05 - 6 Evapor#F7A08
Uploaded by
cymyCopyright:
Available Formats
Evaporation of a Binary Mixture
This is the problem in Figures 5.4 and 5.5. The following parameters are given:
benzene (1), bulk mole fraction
toluene (2), bulk mole fraction
Interfacial equilibrium
Mass transfer coefficient
Concentration of liquid
The total flux
x1 := 0.5
x2 := 1 x1
y2 = K2 x2
k12 := 10
c := 10
y1 = 1 y2
K := 0.5
N := 1 k12 c
N = N1 + N2
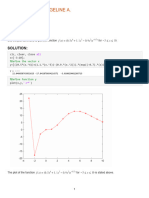
You are to compute the two fluxes and the composition of the gas leaving the drop.
This problem is more complicated than the the previous ones because you do not know
the interface compositions. This means that you have the following unknowns:
x1 , x2 , y1 , y2 , x1 , x2 , N1 , N2
So eight unknowns, which requires eight equations. We begin by making estimates:
x1 := x1
x2 := 1 x1
x1 := 0.5 ( x1 + x1)
y2 := K x2
x2 := 0.5 ( x2 + x2)
y1 := 1 y2
N1 := 0.9 N
N2 := 0.1 N
Below we have constructed the greater part of the Given..Find block for you. It begins
with a 'summation equation' for the two unknown mole fractions (often a useful equation),
calculation of the average mole fractions in the film (which are auxiliary unknowns) and
the vapour compositions.
You see that you still need three more equations. Here are two hints to find these.
(a) There are two transport relations in Figure 5.5. Are these independent?
(b) What do you know about the ratios and sum of the fluxes?
Given
x1 + x2 = 1
x1 = 0.5 ( x1 + x1)
x2 = 0.5 ( x2 + x2)
Answer := Find ( x1 , x2 , y1 , y2 , x1 , x2 , N1 , N2 )
N1 := Answer6
N1 = 0.9
N2 := Answer7
N2 = 0.1
x1 := Answer0
x1 = 0.5
x2 := Answer1
x2 = 0.5
y2 = K x2
y1 = 1 y2
we have put the result in
the vector 'Answer'
note that the subscripts of
'Answer' begin with 0
You might also like
- Assignment 2 SolutionsDocument10 pagesAssignment 2 SolutionsNengke LinNo ratings yet
- Real Analysis Questions and SolutionsDocument2 pagesReal Analysis Questions and SolutionsJJ75% (16)
- MIT15 053S13 Ps6solDocument8 pagesMIT15 053S13 Ps6solErkin KorayNo ratings yet
- Solution Key Comprehensive Question PaperDocument8 pagesSolution Key Comprehensive Question PaperADITYA K RNo ratings yet
- Sequences and Limits: July 31, 2007 13:35 WSPC /book Trim Size For 9in X 6in Real-AnalysisDocument14 pagesSequences and Limits: July 31, 2007 13:35 WSPC /book Trim Size For 9in X 6in Real-Analysissan_kumar@ymail.commNo ratings yet
- LECTURES 30 36: Receptor Ligand Binding: Problem 1 Problem 1Document8 pagesLECTURES 30 36: Receptor Ligand Binding: Problem 1 Problem 1Rojan PradhanNo ratings yet
- Solutions To Assignment 2: Problem 1: Smallest Error in DifferentiationDocument3 pagesSolutions To Assignment 2: Problem 1: Smallest Error in DifferentiationsivasaipranavjNo ratings yet
- Variation of Parameters Malam JawziDocument8 pagesVariation of Parameters Malam JawziismailhashimyusufNo ratings yet
- More Proofs of The Diverge of The Harmonic SeriesDocument18 pagesMore Proofs of The Diverge of The Harmonic SeriesCrystal BakerNo ratings yet
- Difference Calculus and Generating Functions. Santos, D. A. 2005Document34 pagesDifference Calculus and Generating Functions. Santos, D. A. 2005José Daniel Rivera Medina100% (1)
- CH 10Document83 pagesCH 10이잉No ratings yet
- Student Id: Answer Key: Applied Math 245 Midterm Exam: Winter 2007Document7 pagesStudent Id: Answer Key: Applied Math 245 Midterm Exam: Winter 2007RobbenNo ratings yet
- MTH401 Subjective Solved Mid Term Past PaperDocument27 pagesMTH401 Subjective Solved Mid Term Past PaperChugtahi100% (1)
- MTH401 Allcurrentsubjectivesolvedcorrectly 2013 and 2012Document27 pagesMTH401 Allcurrentsubjectivesolvedcorrectly 2013 and 2012Muhammad HanzalaNo ratings yet
- EEE 3104labsheet Expt 02Document5 pagesEEE 3104labsheet Expt 02Sunvir Rahaman SumonNo ratings yet
- Cive162001 2006 Brief SolutionsDocument7 pagesCive162001 2006 Brief Solutionssjeyarajah21No ratings yet
- Famous InequalitiesDocument29 pagesFamous InequalitiesG100% (2)
- Exercises Computational Fluid Dynamics: General RemarksDocument2 pagesExercises Computational Fluid Dynamics: General RemarksJulio Cesar ChavezNo ratings yet
- Examples of The Solutions of Differential Fluid Mechanics ProblemsDocument12 pagesExamples of The Solutions of Differential Fluid Mechanics ProblemsBrandon HoldenNo ratings yet
- TFY4305 Solutions Extra ExercisesDocument24 pagesTFY4305 Solutions Extra ExercisesSelim Echeverría AleuyNo ratings yet
- AP PDX and IndexDocument29 pagesAP PDX and IndexPamela SotoNo ratings yet
- HO6 HW2SolutionsDocument11 pagesHO6 HW2SolutionsJackNo ratings yet
- Ultimo 10 Supch03 PDFDocument15 pagesUltimo 10 Supch03 PDFmri_leonNo ratings yet
- Midterm1 SampleDocument4 pagesMidterm1 SampleChelsea ClarkNo ratings yet
- N +1 N N ( 1) NDocument3 pagesN +1 N N ( 1) NImelda RozaNo ratings yet
- Math3160s13-Hw9 Sols PDFDocument4 pagesMath3160s13-Hw9 Sols PDFPei JingNo ratings yet
- DM515 - Introduction To Linear and Integer Programming: Sheet 1, Spring 2010Document3 pagesDM515 - Introduction To Linear and Integer Programming: Sheet 1, Spring 2010Gustavo G Borzellino CNo ratings yet
- Matlab Code 3Document29 pagesMatlab Code 3kthshlxyzNo ratings yet
- Vcsms Prime: 0 N N 1 N 1 N 1 0 0 5Document2 pagesVcsms Prime: 0 N N 1 N 1 N 1 0 0 5marc7victor7salesNo ratings yet
- Balticway 11 SolDocument20 pagesBalticway 11 Solbenedito freireNo ratings yet
- IMO 2005 NotesDocument12 pagesIMO 2005 NotesAmenElleh ShilNo ratings yet
- Math 171 Solutions To Homework Problems Spring 2005Document2 pagesMath 171 Solutions To Homework Problems Spring 2005lusienopopNo ratings yet
- BV Cvxbook Extra ExercisesDocument237 pagesBV Cvxbook Extra ExercisesWilian GuamánNo ratings yet
- Trigonometry and Complex Numbers: Jubayer Rahman Nirjhor Class 10, Annada Govt. High School, BangladeshDocument5 pagesTrigonometry and Complex Numbers: Jubayer Rahman Nirjhor Class 10, Annada Govt. High School, BangladeshToaster97No ratings yet
- Must Seeeeee AnsDocument16 pagesMust Seeeeee AnsNeda'a HamedNo ratings yet
- Practice Final1 EE235Document9 pagesPractice Final1 EE235Phan Phuong NgocNo ratings yet
- Exam 1 Rev SOLDocument12 pagesExam 1 Rev SOLmexefon390No ratings yet
- Numeths Final ExamDocument2 pagesNumeths Final ExamJef MacatugobNo ratings yet
- Assignment 3Document1 pageAssignment 3Rojan PradhanNo ratings yet
- Binomial ExpansionsDocument4 pagesBinomial ExpansionsVictor CrisostomoNo ratings yet
- EEE 3105: Signals and Linear Systems: K N H K X N yDocument16 pagesEEE 3105: Signals and Linear Systems: K N H K X N yMozammel HossainNo ratings yet
- Basel Problem ProofDocument7 pagesBasel Problem ProofCody JohnsonNo ratings yet
- Math 5310, Homework #5: Problem 1Document11 pagesMath 5310, Homework #5: Problem 1rashslashNo ratings yet
- 102 Sol 1102Document10 pages102 Sol 1102api-3701114No ratings yet
- ECE 305 Homework: Week 7: V K T Q N N NDocument10 pagesECE 305 Homework: Week 7: V K T Q N N NHayden FongerNo ratings yet
- Binomial TheoremDocument9 pagesBinomial TheoremIan Lester Toledo100% (1)
- Trigonometry and Complex Numbers: Jubayer Rahman Nirjhor Class 10, Annada Govt. High School, BangladeshDocument5 pagesTrigonometry and Complex Numbers: Jubayer Rahman Nirjhor Class 10, Annada Govt. High School, BangladeshJubayer Rahman NirjhorNo ratings yet
- Sloane 5Document34 pagesSloane 5VipulNo ratings yet
- Keller Box PaperDocument20 pagesKeller Box Paperfareeha KhanNo ratings yet
- A) Current Flowing Across The PN: KT D S QDocument4 pagesA) Current Flowing Across The PN: KT D S QjohnNo ratings yet
- Assignment 4 SolutionDocument14 pagesAssignment 4 SolutionnprashantshekharNo ratings yet
- 4 Problem Set 4 - BifurcationsDocument6 pages4 Problem Set 4 - BifurcationsAugusto CabreraNo ratings yet
- SolutionsDocument16 pagesSolutionsChaitanya PattapagalaNo ratings yet
- 2016 Fall Estimation Theory Midterm I SolDocument7 pages2016 Fall Estimation Theory Midterm I SolbillyNo ratings yet
- Linear Equation (Slope and Y-Intercept)Document21 pagesLinear Equation (Slope and Y-Intercept)AJ SaysonNo ratings yet
- HomeworkDocument8 pagesHomeworkArtianaNo ratings yet
- Math 165: Final Exam - Part 2 Fall 2017: Name: Section #Document8 pagesMath 165: Final Exam - Part 2 Fall 2017: Name: Section #Mohammed IbrahimNo ratings yet
- General Mathematics - M02 - L02 - WEEK 2Document6 pagesGeneral Mathematics - M02 - L02 - WEEK 2Ji PaoNo ratings yet
- Combustion Equilibrium Calculations: A1 A2 A3 A4Document6 pagesCombustion Equilibrium Calculations: A1 A2 A3 A4cymyNo ratings yet
- Counter Current Heat Exchanger CarnahanDocument4 pagesCounter Current Heat Exchanger CarnahancymyNo ratings yet
- Nox Kinetics Calculations - : Cvode Starts at Line 100 On Excel SheetDocument13 pagesNox Kinetics Calculations - : Cvode Starts at Line 100 On Excel SheetcymyNo ratings yet
- Installed Flow CharacteristicsDocument4 pagesInstalled Flow CharacteristicscymyNo ratings yet
- Nox Kinetics Calculations - : Cvode Starts at Line 100 On Excel SheetDocument13 pagesNox Kinetics Calculations - : Cvode Starts at Line 100 On Excel SheetcymyNo ratings yet
- Live Solution Tank ExampleDocument6 pagesLive Solution Tank ExamplecymyNo ratings yet
- Air Standard Cycle - Design Conditions: W - AC (KJ/S)Document6 pagesAir Standard Cycle - Design Conditions: W - AC (KJ/S)cymyNo ratings yet
- Problem 8.6 BubbleDocument10 pagesProblem 8.6 BubblecymyNo ratings yet
- Heat Transfer FinDocument10 pagesHeat Transfer FincymyNo ratings yet
- Problem 8.6 DewDocument10 pagesProblem 8.6 DewcymyNo ratings yet
- Example 8.5a (Vapor Ethane)Document4 pagesExample 8.5a (Vapor Ethane)cymyNo ratings yet
- Example 2.17 BDocument2 pagesExample 2.17 BcymyNo ratings yet
- Problem 10.9bDocument2 pagesProblem 10.9bcymyNo ratings yet
- Problem 8.6 L (160.67F)Document3 pagesProblem 8.6 L (160.67F)cymyNo ratings yet
- Air Standard Cycle With HRSG Supplemental Firing: Overall Energy Balance MethodDocument6 pagesAir Standard Cycle With HRSG Supplemental Firing: Overall Energy Balance MethodcymyNo ratings yet
- Mass Flowrates and Weight %: Styrene FlowsheetDocument1 pageMass Flowrates and Weight %: Styrene FlowsheetcymyNo ratings yet
- Air Standard Cycle - Off Design 2: W - AC (KJ/S)Document8 pagesAir Standard Cycle - Off Design 2: W - AC (KJ/S)cymyNo ratings yet
- Example 8.6c (Vapor Condenser)Document2 pagesExample 8.6c (Vapor Condenser)cymyNo ratings yet
- SI - Real Gas - Design: VariablesDocument9 pagesSI - Real Gas - Design: VariablescymyNo ratings yet
- Linear Data Reconciliation: Narasimhan and Jordache (2000)Document1 pageLinear Data Reconciliation: Narasimhan and Jordache (2000)cymyNo ratings yet
- Example 2.14Document1 pageExample 2.14cymyNo ratings yet
- Example 2.16 ADocument1 pageExample 2.16 AcymyNo ratings yet
- Example 2.16 BDocument1 pageExample 2.16 BcymyNo ratings yet
- Example 6.16aDocument1 pageExample 6.16acymyNo ratings yet
- Air Standard Cycle - Design Conditions: W - AC (KJ/S)Document6 pagesAir Standard Cycle - Design Conditions: W - AC (KJ/S)cymyNo ratings yet
- Feed Reactor in Reactor Out Product Vapor Out Recycle Purge RHSDocument1 pageFeed Reactor in Reactor Out Product Vapor Out Recycle Purge RHScymyNo ratings yet
- Example 2.17 ADocument2 pagesExample 2.17 AcymyNo ratings yet
- 0.25 Reaction N + 3H NH: Ammonia Material Balance Using Gauss Jordan Elimination and Newton RaphsonDocument1 page0.25 Reaction N + 3H NH: Ammonia Material Balance Using Gauss Jordan Elimination and Newton RaphsoncymyNo ratings yet
- X (0) X (1) X (2) RHS X X X: Newton-Raphson Method All VBA CodeDocument1 pageX (0) X (1) X (2) RHS X X X: Newton-Raphson Method All VBA CodecymyNo ratings yet
- Example 5.6aDocument1 pageExample 5.6acymyNo ratings yet