Professional Documents
Culture Documents
Fluids and Electrolytes Pathophysiology Nursing
Uploaded by
grad_nurse_2015Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fluids and Electrolytes Pathophysiology Nursing
Uploaded by
grad_nurse_2015Copyright:
Available Formats
Patho Wk 5: Ch.
FLUID DISTURBANCES
Objective: Identify the major consequences/manifestations of abnormal levels of water.
FUNCTIONS OF WATER (its role in our body)
Solvent things can dissolve in H2O
Chemical imbalance altered H2O can lead to altered chemicals
Transports nutrients in/out/to the cell & wastes away from cell
Helps eliminate wastes
Cushions, protects
Lubricates, insulates
WATER = comprises ~60% of the total body weight
ECF+ICF = total body water (sum of all fluids in all compartments)
o Varies with: Age, Weight, & Gender
o Distributed among compartments and spaces moves freely
o Distributed and maintained by osmotic and hydrostatic forces
Intracellular (ICF) = 40% ( ~2/3 of bodys water)
Extracellular (ECF) = 20% ( ~1/3 of bodys water)
o Interstitial = 15% (trans-cellular fluids: lymph, synovial fluid, interstitial fluid, sweat, urine, biliary, hepatic,
CSF, intraocular, peritoneal, pericardial, pleural, etc)
o Intravascular = 5% (in blood/plasma)
WATER REQUIREMENTS
Amount of water necessary to maintain health = 15002500 ml/day
Sources:
o Liquids = 1200 ml; Foods = 1000 ml; Oxidation of food = 300 ml
ROUTES OF WATER LOSS
Kidneys (most) 1-2 liters/day = 1400 ml
Lungs 300-400 ml/day = 300 ml
Skin 0-1000 ml/hr = 600 ml (varies d/t sweating, febrile, etc)
Gastrointestinal tract 100-200 ml/day = 200 ml
WATER LOSS
Sensible
o Urine (MAIN way pee)
o Stool
Insensible
o Lungs breathing
o Skin
OBLIGATORY WATER LOSS
Amt of urine/pee necessary to maintain kidney function = ~300-500 ml/day
Water balance ideally intake = output (happens in euvolemic person)
o 2500 ml intake == 2500 ml output
CAUSES OF WATER LOSS: any work of body or metabolic demand leads to water loss!
Fever (sweating)
Diarrhea, Vomiting
Diaphoresis
Gastric suctioning
Pneumonia ( RR)
Tachypnea
CAUSES OF WATER GAIN
Na+ intake or Na+ retention (Na+ and H2O travel together!)
Patho Wk 5: Ch. 3
Excessive intake of water
Excess secretion of ADH
BODY FLUID COMPARTMENTS
When thinking about labs, the values correlate with ICF vs ECF (would expect high Na+ in ECF, not ICF)
Intracellular (ICF) (40% or ~2/3 of body weight)
o Inside the cells
o Large amounts of K+, PO4 - -, Mg++
Extracellular (ECF) (20% or ~1/3 of body weight)
o Intravascular PLASMA (5% of the body weight)
o Interstitial fluid (15% of the body weight)
o Large amounts of Na+, Ca++, Cl -, and HCO3
Osmosis = movement of H2O DOWN the concentration gradient
HIGH concentrationLOW concentration
Semipermeable membrane Membrane must be more permeable to water
A greater concentration of solutes on one side of the membrane water moves to equal out concentration
Diffusion = movement of SOLUTE molecule from HIGH solute concentration LOW solute concent.
Facilitated Diffusion
Requires a carrier molecule
No energy/ATP required moves HIGHLOW
Ex: Lipid insoluble substances cannot cross plasma membrane (needs glucose carrier)
Active Transport = mvmt of substance across the cell membrane from LOW concentrationHIGH concentration
o ATP is expended
o Ex: Na+/K+ pump
Filtration = mvmt of a fluid through a semipermeable membrane from HIGH pressure area LOW pressure
Hydrostatic Force = the mechanical force of water pushing against cellular membranes
In vascular system hydrostatic pressure = the blood pressure pushing against vascular walls.
Colloid Osmotic Pressure = tendency of plasma proteins to hold water in the intravascular spaces (maintains water in ECF)
Albumin is the plasma protein which exerts the greatest osmotic pressure
o Albumin is formed in the liver
o Most abundant plasma protein
o Binds to hormones and transports them
o Acid-base balance
Globulin
o Antibodies (IgG)
o Humoral immunity
o Transport of iron and fats
Fibrinogen
o Blood coagulation/clotting cascade
Osmolality = a measure of the concentration of molecules per kilogram of water (mOsm/kg weight measurement)
*PREFERRED measure of osmotic activity in clinical assessment
Indicates hydration status, body fluid concentrations
Can measure serum or urine osmolality
Normal osmolality = 285-295 mOsm/kg
Osmolality = 2 (Na) + K + BUN + Glucose
3
18
Patho Wk 5: Ch. 3
Osmolarity = a measure of the concentration of molecules per liter of solution (mOsm/L)
NOTE: Difference btwn osmolality and osmolarity matters what types of solutes you are measuring
o Na & K vs. proteins, glucose lipids present also
Tonicity = describes the effective osmolality of a solution (term interchangeable with osmolality !!)
ISOtonic Solutions has the SAME osmolality as the ECF
Examples
o D5W
Distributes evenly in the body compartments
Used to replace deficits of total body water
o Normal Saline (0.9% NS)
NOTE: Each liter adds liter to ECF & adds liter to interstitial fluid
HYPERtonic Solutions have a HIGHER concentration of solute and are MORE CONCENTRATED than ECF
Examples
o 3% saline
o 5% saline
Net movement (PIC) = ICF ECF
HYPOtonic Solutions have a LOWER concentration of solute and are MORE DILUTE than ECF
Examples
o 1/2 NS
o 1/4 NS
Net movement (PIC): ECF ICF
THIRST = A desire for water
Regulated by osmoreceptors in the hypothalamus
Angiotensin II plays a role
Symptom: Dryness of the mouth
CAUSES:
O d OSMOLARITY
Hypertonicity occurs (Na+ higher)
Water is drawn from the cell (crenation)
Water is ingested Cell returns to normal
O
d FLUID VOLUME
Loss of fluid (ex hemorrhage)
d circulation (CHF: H2O moves from ICF to ECF look swollen but actually depleted)
Dryness of the mouth Thirst triggered replace fluids resolves
ALTERED THIRST MECHANISMS:
o Coma dont know theyre thirsty
o Senility (dementia), Psychosis, Confusion cant comprehend thirst
o Psycogenic Polydipsia drink way too much water water intoxication, hypoNa+
REGULATION OF BODY FLUID KIDNEYS!! (review in book)
Filtration r/t hydrostatic pressure
Secretion Mvmt from blood to renal tubules
Reabsorption Mvmt from renal tubules to the blood
Excretion - Mvmt from kidney to the environment
KIDNEYS how well are they working?
o Glomerular filtration rate (GFR) rate of urine production
indicates stage of kidney dz
o Renal perfusion
Cardiac output ( CO, hypoTN - s renal perfusion)
Patho Wk 5: Ch. 3
o
o
o In shock state, body shunts blood to heart/brain 1st
Ex: Renal artery stenosis
Hydration status Dehydration not filtering or sending water/fluids to kidneys
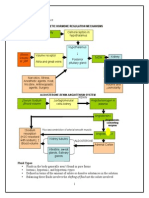
RAAS SYSTEM
*Renin-Angiotensin-Aldosterone System* (RAAS) negative feedback loop!
Regulates BP, fluid status, everything!
STIMULUS = BP, GFR, Na+, or renal perfusion
o stimulates kidneys to release renin
o stimulates conversion of angiotensin I to
angiotensin II via ACE
o tells BP to rise tells adrenal gland to produce
aldosterone
o aldosterone tells kidneys to hold onto Na+ (and in
turn, H2O), so BP rises
ALDOSTERONE = mineralocorticoid (steroid) hormone
Produced and secreted by the adrenal cortex
GOAL = conserve Na+, restore blood volume and H2O
Promotes Na+ & H2O reabsorption by the proximal tubule in the kidney
Stimulates secretion/excretion of K+ by the distal tubule of the kidney ( thus ing K+ in the ECF)
Aldosterone = End product of RAAS system!!
o Stimulus received (DECd BP, renal blood flow, Na+)
o Renin secreted by juxtaglomerular cells of the kidney stimulates formation of angiotensin I.
o ACE (angiotensin converting enzyme) in the pulmonary vessels converts Angiotensin I to II.
o 2 functions of angiotensin II: vasoconstriction (elevates BP) + stimulates the secretion of aldosterone
o When blood volume is restored, feedback loop turned off inhibits renin release.
Causes of d secretion
o HypoNa+
o HyperK+ (must get rid of it!)
o Activation of the RAA
Causes of d secretion
o HyperNa+
o HypoK+
Patho Wk 5: Ch. 3
Inhibition of the RAA mechanism
ANTIDIURETIC HORMONE (ADH) [arginine vasopressin (AVP)]
Formed in the supraoptic and paraventricular nuclei of the hypothalamus
Released by posterior pituitary
FUNCTION = INCs H2O reabsorption in the distal convoluted tubule and
collecting ducts of the kidneys
O tells kidney to hold onto more H2O!
PROCESS:
o d plasma osmolality d/t d H2O or d Na+
o Stimulates osmoreceptors in hypothalamus which tells post.
pituitary to release ADH
o ADH tells kidneys to permeability of renal tubular cells to water
o restores plasma volume, BP rises
o move towards euvolemic state
Factors INHIBITING RELEASE of ADH (s ADH)
o Hypotonicity of the ECF (d osmolality)
o Ethanol
o INCd ICP (intracranial pressure)
Factors STIMULATING RELEASE of ADH (s ADH)
o Hyperosmolality of the ECF
o Hypovolemia (ex bleeding)
o HypoTN
o d body temperature ( dehydration d/t fever)
o Medications:
Narcotics
Antineoplastics
Oral hypoglycemic
Beta adrenergic drugs
o Stress, Pain
o Trauma
Anything that makes body work harder!!
o Surgery
PROSTAGLANDINS = Fatty acids widely distributed in the cells of the body
MOA: Interferes with the renal tubules response to ADH
BODY FLUID REGULATION VIA:
o s Na+ & H2O excretion
o Renal vasodilation to protect kidney from ischemic injury
FUNCTIONS:
o Inflammatory process
o Blood pressure
o Uterine contractions
o Increased GI motility
o Bronchoconstriction
GLUCOCORTICOIDS (CORTISOL)
Hormones produced by the adrenal glands
Promotes Na+ and H2O retention (weak effect)
Cushings Syndrome:
o Exogenous (outside body)
o Endogenous (problem with cortisol excretion)
Patho Wk 5: Ch. 3
S/S: Edema, HTN (volume overloaded), HyperNa+, HyperK+
ETIOLOGY = Excess cortisol (which helps regulate blood sugar)
ATRIAL NATRIURETIC PEPTIDE (ANP)
Released from myoendocrine cells in the atria
STIMULUS = atrial stretch (d/t d atrial pressure, intra-atrial volume, CHF, volume overload, etc)
o Turned off when atrial pressure lowers (a negative feedback loop)
PHYSIOLOGIC EFFECTS:
o s Na+ & H2O excretion by kidneys
o Renal vascular dilation
o Antagonist of RAAS Inhibits release of aldosterone & ADH
ALTERATIONS IN WATER BALANCE
ISOTONIC FLUID IMBALANCE changes in total body water are accompanied by proportional changes in electrolytes
Na+ or H2O or in the SAME proportion
Na+ and osmolality are WNL
Types: Hypovolemia, Hypervolemia
HYPOVOLEMIA = Fluid Volume DEFICIT (contraction of ECF)
O Na+ or H2O in the SAME proportion
o
CAUSES:
Inadequate intake
GI/GU losses
Skin losses
Third-space losses
SIGNS (classic sign of dehydration):
d Body weight
Mild deficit = 2% loss
Moderate deficit = 4% loss
Severe deficit = 6% loss
d UOP
Oliguria little bit
Anuria NO urine
d Urine and Serum osmolality (more concentrated)
Poor skin turgor
Dry mucous membranes
Postural hypotension
d HR (Tachycardia)
MAJOR COMPLICATIONS:
d CO
Shock, Death
HYPERVOLEMIA = Fluid volume EXCESS (ECF excess)
O Na+ or H2O in the SAME proportion
O
CAUSES:
Patho Wk 5: Ch. 3
Excessive administration of IV NS
Over-secretion of aldosterone (kidneys retain Na+ and H2O)
Drug effect of cortisone
Renal failure
Results in :
in capillary pressure
in oncotic pressure
in serum aldosterone
SIGNS/SYMPTOMS:
Acute weight gain
Edema, HTN
Pulmonary edema
SOB, Crackles, Dyspnea, Cough
Venous distention
d HCT, d plasma protein concentration (dilution)
MAJOR COMPLICATIONS:
Pulmonary edema BAD!
**Pink, frothy sputum
Hypoxia, Dyspnea, SOB, Pleural effusion
HR
EDEMA = Expansion or accumulation of interstitial fluid volume (ECF)
First-space cellular
Second-space intravascular
Third-spacing interstitial (ex swelling in ankles, ascites in abd)
o
CAUSES:
o d hydrostatic force
o d colloid osmotic pressure (low albumins, nephrotic syndrome, end-stage liver failure)
o d capillary permeability (sepsis)
o Obstruction of a lymphatic vessel
o Na+ and H2O excess
DISTRIBUTION of Edema: (mostly r/t condition of pt)
o Localized = Venous or lymphatic obstruction (lymphedema d/t mastectomy)
o Generalized = Hypoproteinemia (low protein/low albumin states)
o Dependent = Heart failure (d EF)
Edema Terms:
o Ascites = Fluid within the peritoneal cavity (abdomen)
o Pleural effusion = Fluid within the pleural space
o Pericardial effusion = Fluid within the pericardium
o Pulmonary edema = Fluid within the alveoli
CONCEPTS R/T FLUID INTAKE
All foods which are liquids at room temperature fluid!
One fluid ounce = 30cc or 30 ml
Fever (insensible fluid loss)
o 101o 103o: s 24-hr fluid needs by at least 500 ml
o >103o: s 24-hr fluid needs by at least 1000 ml
Normal urinary output = = 1 ml/kg/hr (1 ml of urine per kilogram of body weight per hour)
o Anuria = NO urine output
o Oliguria = urine output
Patho Wk 5: Ch. 3
Polyuria = urine output
CONCEPTS R/T FLUID OUTPUT
Includes: Urine, Vomitus, Diarrhea, Drainage (ex: chest tube, abdominal fistula), Perspiration
CONCEPTS R/T FLUID VOLUME DEFICIT
d UOP
d Urine specific gravity (i.e. high urine osmolality more concentrated d/t body holding onto water)
Poor skin & tongue turgor
Dry mucous membranes
d or absence of tearing and/or salivation
d Body weight
d Thirst (if intact thirst mechanism)
Skin may be warm and flushed in moderate FVD
Skin may be cold and clammy in severe FVD
d intraocular pressure
Sunken fontanels
getting worse, entering SHOCK STATE, body attempting to compensate for FVD:
HR, BP, CVP
Neck & Hand veins flat
o What happens to CO and perfusion?? Both suffer if not corrected/reversed
BP and warm is better than BP and cold (means not perfusing extremities)
TYPES OF FLUID REPLACEMENT
Crystalloids
o Dextrose (D5, D5 NS)
o Saline (0.9% NS, NS, NS, 3%, 5%)
Colloids (Proteins)
o Albumin Plasma protein fraction
Colloid substitutes (volume expanders)
o Dextran
o Hetastarch
Blood transfusions
o Packed red blood cells (PRBCs)
o Whole blood
DIURETICS: GETTING RID OF FLUID (can also limit fluid intake! Duh.)
Thiazide Diuretics
o Actions:
Inhibit Na+ resorption in the kidney (thus, getting rid of water)
Causes LOSS of Na+, Cl-, Mg++ and K+
s ECF volume
o Examples:
Chlorothiazide (Diuril)
Hydrochlorothiazide (HCTZ, Esidrix)
Loop Diuretics
o Actions:
Acts in the limbs of Henles loop
Causes LOSS of Na+, Cl-, and K+
s ECF volume
o Examples:
Furosemide (Lasix)
Patho Wk 5: Ch. 3
Ethacrynic acid (Edecrin)
Bumetanide (Bumex)
Potassium-Sparing Diuretics (weaker diuretic effect)
o Actions
Inhibit Na+ reabsorption
Cause LOSS of Na+, K+ (lose some K but not as much)
Cause an in Clo Examples:
Spironalactone (Aldactone)
Amiloride
Triamterene (Dyrenium)
Osmotic Diuretics
o s osmotic pressure and causes intracellular and interstitial fluids to enter the vascular spaces
o *Used frequently in neuro for d ICP
o Actions:
s Na+ and Cl- Hyponatremia, Hypochloremia
s ECF volume (VOLUME EXPANDER)
o Example: Mannitol
ELECTROLYTE DISTURBANCES
SODIUM (NA+) == 135 145 mEq/L
Main extracellular cation
FUNCTIONS:
o Maintains osmolality
o Participates in active transport (Na/K ATP pump)
o Helps regulate body fluids
o Participates in the action potential
o **where sodium goes, water will follow**
HYPONATREMIA: HYPO-OSMOLAR IMBALANCE
LABS: d serum osmolality, chloride, hematocrit, and BUN (dilution)
*Most common electrolyte imbalance in hospitalized patients
Cellular effect
o Cells become swollen (Na+ and H2O move INTO cell)
o Amt of Na+ has d inside the cell
CAUSES of ABSOLUTE hyponatremia ( pure Na+ deficit)
o Extra-renal losses: Sweating, GI losses (V/D), GI suctioning, burns,
o Diuresis
o Adrenal insufficiency
CAUSES of RELATIVE hyponatremia ( dilutional water exceeds Na+)
o Excess intake of plain water
o Tap water enemas
o Absorption of water from irrigating fluids used in a TURP (trans-urethral prostectomy)
Patho Wk 5: Ch. 3
o
o
10
Long-term IV therapy with D5W
d ADH levels
SIADH Syndrome of Inappropriate Anti-Diuretic Hormone
o Inappropriate in ADH secretion retain H2O FVE !
o ETIOLOGY: acute infections, brain trauma, surgery, ADH-secreting tumors
o
CAUSE: NOT excess H2O intake, but d renal reabsorption of H2O d/t inappropriate ADH secretion
Note: ADH normally secreted in hyperosmolar or hypovolemic state
Secretion is stimulated by factors other than the norm
SIGNS/SYMPTOMS:
Blood #s - dilutional effect Na+ (kidneys continue to excrete), serum osmolality
Urine #s - urine Na+ and urine osmolality
Volume: blood vol s, UOP s, Concentrated urine
Common underlying disorders:
Tuberculosis
Pneumonia
Cancer: Oat cell carcinoma, Pancreatic CA
Encephalitis
Meningitis
Some medications
MAJOR COMPLICATIONS: THINK NEURO
o *Neurological disturbances
o Cerebral edema
o Headache, lethargy
o Depression, confusion
o Seizures, Convulsions, Coma
o Cardiovascular disturbances
Postural hypotension
Shock
HYPERNATREMIA: HYPER-OSMOLAR IMBALANCE
Cellular effect
o Cells shrink; Cellular dehydration
o d Na+ levels outside the cell
CAUSES: (acute Na+ or loss of H2O) (NOTE: if both occur, will have ICF and ECF dehydration)
o Excessive oral or parenteral (IV) Na+ intake
o Near-drowning in salt water
o Hypertonic tube feedings without free water
o Inability to respond to thirst
o d water losses
o Diabetes insipidus (DI)
o Over-secretion of aldosterone (primary hyper-aldosteronism)
o Cushings syndrome excess secretion of ACH
MANIFESTATIONS: (DRY!)
o Thirst; Dry, sticky mucous membranes
o Tongue dry and swollen
o d temperature
o Agitation, Confusion
o Convulsions
o d HR
o d UOP (Oliguria or anuria)
o d urine specific gravity
Patho Wk 5: Ch. 3
11
MAJOR COMPLICATIONS:
o Net osmotic diuresis!!
o Water (& Na+) moves out of the cells into the circulation/ECF hypervolemia/FVE
o Cellular dehydration
o Circulation s
o Cell function is impaired
o Brain cells are particularly susceptible
o *Neurologic changes EARLY sign!
POTASSIUM (K+) == 3.5 to 5.0 mEq/L
Main intracellular cation
FUNCTIONS:
o Transmission of nerve impulses
o Resting membrane potential
o Acid-base balance
o Promotes myocardial, skeletal, and smooth muscle contractility
FACTORS AFFECTING REABSORPTION/SECRETION OF K+
o Sodium balance Na+ deficit results in d K+ loss (inverse relationship)
o Acid-base balance:
Acidosis results in excretion of H+ and retention of K+
Metabolic acidosis treating acidosis will K+ - i.e. must lower the CO2
Alkalosis results in retention of H+ and excretion of K+
o Use of diuretics Results in d K+ loss
o Loss of gastric secretions Result in d K+ loss
o
o
o
o
Aldosterone = promotes K+ excretion
Epinephrine = promotes K+ reabsorption
Anabolism = K+ enters the cell
Catabolism = K+ leaves the cell
Movement of K+ INTO the cell is dependent on:
Oxygen
Tx hyperkalemia by giving insulin and glucose moves K+ INTO cell
Glucose
Insulin
HYPOKALEMIA == <3.5 mEq/L
ETIOLOGY:
o Poor nutrition
o Diuretic therapy ***
o Vomiting, diarrhea, GI suctioning
o Burns
o Alkalosis
o Hyperaldosteronism
MAJOR COMPLICATIONS: (THINK muscle contractility, action potential and ATP pump)
o Cardiovascular T wave abnormalities
Dysrhythmias, hypoTN, digitalis toxicity exacerbation, myocardial damage, cardiac arrest
o Neurological
Lethargy, confusion, depression
o Gastrointestinal
Paralytic ileus
o *Skeletal muscle MAJOR!
Weakness, flaccid paralysis, weakness of respiratory muscles, respiratory arrest
o *Renal system
d ability to concentrate urine, water loss, kidney damage
o *Acid base balance
Patho Wk 5: Ch. 3
12
Metabolic alkalosis
HYPERKALEMIA == >5.0 mEq/L
ETIOLOGIES:
o Taking in too much K+ & kidneys unable to compensate
o Retention of K+
Renal failure, potassium-sparing diuretics, adrenocortical insufficiency
o *Cell lysis = Major release of K+ from the cells
Trauma, burns, transfusions of old blood, metabolic acidosis
o Rapid IV K+ administration
TREATMENT: insulin & glucose (acute tx), calcium chloride, albuterol, kayexalate (takes longer)
MAJOR COMPLICATIONS: can kill!
o Nervous system
Paraesthesia
o Neuromuscular
Muscle twitching, muscle weakness, paralysis
o Cardiovascular
Bradycardia
Cardiac arrest
*Peaked T waves on EKG
Tombstone waves dead heart
CALCIUM (CA+) == 8.5 to 10.5 mg/dl or 4.5 to 5.8 mEq/L
FUNCTIONS:
o Formation of bone and teeth
o Contraction of muscle
o Blood coagulation
o BLOCKS Na+ transport INTO the cell
o Transmission of nervous impulses
Three Types of Calcium:
o Bone
o Ionized Calcium
o Protein Bound (*primarily to albumin)
REGULATION OF CALCIUM negative feedback loop
o Parathyroid hormone (PTH)
Bone to plasma
Intestinal absorption
o Calcitonin
Patho Wk 5: Ch. 3
13
Plasma to bones and teeth
Calcitrol (activated vitamin D 25-hydroxy Vit D)
Calcium absorption from intestines
Aids in mobilization of bone calcium to where it is needed
CORRECTING TOTAL CALCIUM LEVELS FOR ALBUMIN
o If calcium level, must check albumin level!
If albumin level normal correct calcium deficit
If albumin level low correct albumin deficit
o *When total calcium is the only calcium level you have, the level has to be corrected for albumin level
o *CALCULATION: For every 1 gram in albumin below normal level, the measured total serum
calcium should be adjusted up by 0.8 mg/dl
EXAMPLE of Calcium Correction:
o Ca+ = 7.0
o Albumin = 2.3
o Normal albumin = 4
o 4 2.3 = 1.7(.8) = 1.36
o 7.0 + 1.36 = 8.36 Corrected Calcium
= 8.0
= 2.0
4 2 = 2(.8) = 1.6
8.0 + 1.6 = 9.6 corrected
o
thus, Ca level not as low as you thought!
HYPOCALCEMIA
ETIOLOGIES: (think of feedback loop!)
O Hormone-dependent (body doesnt know that it needs to keep calcium)
Hypoparathyroidism (d PTH)
d calcitonin
Vitamin D deficiency (d vit D)
o Renal-dependent
Renal failure
Hyperphosphatemia (not excreting phos from kidneys)
Loop diuretics
o Acid-base imbalance
Alkalosis
o Nutritional
Malnourishment; Malabsorption syndromes (intestinal)
Multiple blood transfusions (d/t preservative)
Neonatal hypocalcemia
o Deposition of ionized calcium in bone or soft tissue
*SPECIAL SIGNS OF HYPOCALCEMIA (PIC)
o Trousseaus sign
Inflate BP spasm of hand, wrist, fingers
o Chvosteks sign
Tap on side of face in front of earlobe twitching
MAJOR COMPLICATIONS:
o Nervous system
Paraesthesia
o Muscular system LATE signs
Tetany, laryngeal spasms
o Cardiovascular system (PIC)
Congestive heart failure
d CO
Cardiac dysrhythmias r/t QT interval (hyper = shortened, hypo = elongated)
TREATMENT:
o Oral calcium TUMS
o Vitamin D replacement
o IV Calcium gluconate (preferred)
Patho Wk 5: Ch. 3
o
o
o
14
IV Calcium chloride (VERY toxic to vessel need central line)
IV Calcium gluceptate
Thiazide diuretics
HYPERCALCEMIA == >12.5
ETIOLOGIES:
O Hormone imbalance
Hyperparathyroidism (NOTE: 4 parathyroid glands near thyroid)
Vitamin D excess
o Thiazide diuretic use
o *Bony cancers, malignancies
o Sarcoidosis in lungs
o Resorption from bone
Prolonged immobilization
Multiple fractures
Bone tumors
Multiple myeloma
MAJOR COMPLICATIONS:
o Neurological manifestations
Lethargy, confusion, coma
o Skeletal manifestations
Deep bone pain
Pathological fractures
o Renal manifestations
*Kidney stones, renal failure
o Gastrointestinal manifestations
Constipation
Anorexia
Nausea and vomiting
o Cardiovascular manifestations
Shortened QT interval
Bradycardia
Cardiac arrest
TREATMENT Dilute the calcium!
o Loop diuretics & IVF 1st line therapy
o Oral or IV phosphate preparations
o Plicamycin or Calcitonin (slows bone resorption)
MAGNESIUM (MG+) == 1.5 to 2.5 mEq/L
Primarily regulated by PTH
Interacts with Calcium at cellular level!
FUNCTIONS:
o Aids in neuromuscular transmission
o Aids in heart muscle contraction
o Activates enzymes for cellular metabolism of carbohydrates and proteins
o Aids in transmission of hereditary information to offspring
HYPOMAGNESEMIA
ETIOLOGIES:
o Malnutrition
o Chronic alcoholism
o Loop diuretics
o Diarrhea
o Severe dehydration
o Malabsorption syndrome (Crohns disease)
Patho Wk 5: Ch. 3
MANIFESTATIONS:
o Severe respiratory muscle depression
o Apathy, depression, confusion
o Muscle weakness, tremors, tetany
o Life-threatening cardiac arrhythmias (PIC)
TREATMENT:
o IV Magnesium sulfate
2 Grams over 2-3 hrs/ 4gms over 4 hours
12 Grams over 12 hours
HYPERMAGNESEMIA (rare; often r/t renal failure)
ETIOLOGIES:
o Chronic renal failure
o Laxatives or Antacids that contain magnesium
MANIFESTATIONS:
o Severe muscle weakness
o Depression of respirations
o Inability to swallow
o Hyporeflexia
o Hypotension
o Cardiac dysrhythmias
TREATMENT:
o 1st d/c all magnesium-containing drugs
o Fluid therapy
o Calcium gluconate to counteract the effects of magnesium
o Mechanical ventilation if needed
PHOSPHORUS == 2.7 to 4.5 mg/dl
FUNCTIONS:
o Aids in structure of cellular membrane
o Contributes to the metabolism of glucose, fat, and protein
o Helps maintain bone hardness
o Acid-base balance
HYPOPHOSPHATEMIA
ETIOLOGIES:
o Alcoholism
o Malnutrition
o Diabetic ketoacidosis (DKA)
o M/C intestinal malabsorption or INC renal excretion of phos
MANIFESTATIONS:
o Hemolytic anemia
o Muscular weakness (respiratory esp)
o Paraesthesia
o GI distress d/t reduced energy and oxygen stores in cells
TREATMENT:
o Fleets Phospho-soda enema
o IV or oral phosphate
HYPERPHOSPHATEMIA
ETIOLOGIES:
o *Chronic renal failure
o Rapid cell catabolism
o Excessive intakes of phosphates
TREATMENT:
15
Patho Wk 5: Ch. 3
o
o
o
o
o
o
16
Dietary restriction of phosphates
Aluminum-containing antacids
Hydration
Correct hypocalcemia
Renagel , Phosrenol (non-calcium-based phosphate binders bind phos with meals & excrete in stool)
Tums, Phoslo (calcium-based phosphate binders bind & excrete in stool)
You might also like
- Fluids & ElectrolytesDocument15 pagesFluids & Electrolytesarvinnnn100% (2)
- Fluid and Electrolytes (PDF File) : A. Body FluidsDocument5 pagesFluid and Electrolytes (PDF File) : A. Body FluidsLegendX100% (3)
- Nursing Fluids and ElectrolytesDocument14 pagesNursing Fluids and Electrolytesaga1028100% (18)
- Endocrine NursingDocument2 pagesEndocrine NursingUnclePorkchop94% (34)
- Mabes Fluid and Electrolyte ImbalancesDocument15 pagesMabes Fluid and Electrolyte ImbalancesMabesNo ratings yet
- Fluid & ElectrolyteDocument26 pagesFluid & Electrolytesanjana bhatia100% (1)
- Handout Medical-Surgical Nursing Fluid and ElectrolyteDocument13 pagesHandout Medical-Surgical Nursing Fluid and ElectrolytePaul Christian P. Santos, RN100% (10)
- NCLEX Fluids & ElectrolytesDocument6 pagesNCLEX Fluids & ElectrolytesNathalee Walker100% (1)
- Fluids and Electrolytes ExamDocument3 pagesFluids and Electrolytes Exammonmon100% (3)
- Fluids and Electrolytes HandoutsDocument15 pagesFluids and Electrolytes HandoutsSiv Carlaisle100% (3)
- Fluid, Electrolyte and Acid-Base Homeostasis (Renal)Document36 pagesFluid, Electrolyte and Acid-Base Homeostasis (Renal)xarae23No ratings yet
- Chapter 14 Fluid and Electrolytes ChartsDocument7 pagesChapter 14 Fluid and Electrolytes ChartsBNA_RN100% (3)
- Electrolytes StudyDocument14 pagesElectrolytes StudyAlly Shawnae Ordanozo88% (8)
- Neurology Nursing Study GuideDocument19 pagesNeurology Nursing Study GuideHayleyLangley100% (5)
- BUN Test Overview and Normal RangesDocument3 pagesBUN Test Overview and Normal Rangesashdmb217100% (49)
- Fluid and ElectrolytesDocument17 pagesFluid and Electrolytesdlneisha61100% (9)
- 313 - Disorders of Renal and Urinary SystemsDocument8 pages313 - Disorders of Renal and Urinary SystemsChrissy Mendoza100% (2)
- Cardiac DrugsDocument10 pagesCardiac Drugssurviving nursing school100% (3)
- Endocrine Review NotesDocument9 pagesEndocrine Review Noteslisette_sakura100% (5)
- Fluids and Electrolytes - RATIODocument16 pagesFluids and Electrolytes - RATIOLouie Bello100% (3)
- Electrolyte ImbalanceDocument2 pagesElectrolyte ImbalanceRanita IvanaNo ratings yet
- Renal ReviewDocument23 pagesRenal ReviewRaven Atisha100% (1)
- HyponatremiaDocument42 pagesHyponatremiaAbdu Raheem100% (1)
- Med SurgDocument98 pagesMed SurgKimsha Concepcion86% (7)
- Fluid and Electrolytes Study GuideDocument16 pagesFluid and Electrolytes Study GuideDianaNursing96% (28)
- Ir-026 Fluid and Electrolytes - 1Document4 pagesIr-026 Fluid and Electrolytes - 1Muhammad ShakeelNo ratings yet
- Nursing Skills: Blood Transfusion/ Iv TherapyDocument6 pagesNursing Skills: Blood Transfusion/ Iv TherapyVince John SevillaNo ratings yet
- Part 1 - Fluid and Electrolyte Balance, Nursing Process, Fluid ImbalancesDocument23 pagesPart 1 - Fluid and Electrolyte Balance, Nursing Process, Fluid Imbalancesfebie pacheco100% (1)
- Fluid and ElectrolytesDocument13 pagesFluid and ElectrolytesHenry Philip93% (15)
- Review Questions Fluid and ElectrolytesDocument2 pagesReview Questions Fluid and Electrolytesmarie100% (13)
- Heart Anatomy, Function, Diseases and TestsDocument62 pagesHeart Anatomy, Function, Diseases and TestsJean Soriano98% (65)
- IV Solution Cheat Sheet: Type Description Osmolality Use MiscellaneousDocument1 pageIV Solution Cheat Sheet: Type Description Osmolality Use Miscellaneousivy_espesoNo ratings yet
- Fluid and Electrolyte Nursing Care Management 112Document7 pagesFluid and Electrolyte Nursing Care Management 112anne marieNo ratings yet
- Myocardial Infarction With CABG Concept MapDocument1 pageMyocardial Infarction With CABG Concept MapMaria Therese100% (1)
- Nursing School Necessities Cheat SheetDocument3 pagesNursing School Necessities Cheat SheetRevNo ratings yet
- Fluid and ElectrolytesDocument13 pagesFluid and ElectrolytesMarcus, RN100% (7)
- Electrolyte ChartDocument2 pagesElectrolyte ChartJenny Varghese100% (4)
- Nursing Pharmacology Drug Study GuideDocument15 pagesNursing Pharmacology Drug Study GuideChelsea SmithNo ratings yet
- Hemodialysi S: Ariane Jake C. Fernandez, RN, MSNDocument37 pagesHemodialysi S: Ariane Jake C. Fernandez, RN, MSNMicah Alexis Candelario100% (3)
- Handout 5 CardioVascular System Overview PDFDocument7 pagesHandout 5 CardioVascular System Overview PDFGrape JuiceNo ratings yet
- IV Solutions CheatsheetDocument1 pageIV Solutions CheatsheetRosemaryCastroNo ratings yet
- Lab Values Cheat SheetDocument8 pagesLab Values Cheat SheetJamie Lebon100% (4)
- Nervous System Nursing CareDocument78 pagesNervous System Nursing CareLouie ParillaNo ratings yet
- Understanding IV FluidsDocument2 pagesUnderstanding IV FluidsJoe Anne Maniulit, MSN, RN100% (5)
- NCLEX - Acid Base Balance Study GuideDocument1 pageNCLEX - Acid Base Balance Study GuideNathalee WalkerNo ratings yet
- COMPREHENSIVE NURSING ACHIEVEMENT TEST (RN): Passbooks Study GuideFrom EverandCOMPREHENSIVE NURSING ACHIEVEMENT TEST (RN): Passbooks Study GuideNo ratings yet
- Chicago Review Press NCLEX-PN Practice Test and ReviewFrom EverandChicago Review Press NCLEX-PN Practice Test and ReviewRating: 4 out of 5 stars4/5 (4)
- The Pathophysiology and Pharmacotherapy of Myocardial InfarctionFrom EverandThe Pathophysiology and Pharmacotherapy of Myocardial InfarctionNabil El-SherifNo ratings yet
- Basic of Fluid Therapy ImaDocument69 pagesBasic of Fluid Therapy Imal Made ArtawanNo ratings yet
- Week 3Document396 pagesWeek 3Danica Mae BianitoNo ratings yet
- S.No Table of Content Page NoDocument20 pagesS.No Table of Content Page NoTamilArasiNo ratings yet
- Fluids and Electrolyte Balance: Understanding Intracellular and Extracellular Fluid CompartmentsDocument17 pagesFluids and Electrolyte Balance: Understanding Intracellular and Extracellular Fluid CompartmentsSydney DeringNo ratings yet
- Lec. 3 - Fluid and ElectrolyteDocument16 pagesLec. 3 - Fluid and Electrolyteمجيب سلطانNo ratings yet
- BODY Weight 100%: Balance/Imbalances & TherapyDocument11 pagesBODY Weight 100%: Balance/Imbalances & TherapyVictoria Castillo TamayoNo ratings yet
- Fluids and Electrolytes-2Document82 pagesFluids and Electrolytes-2Jem Loterte100% (1)
- Electrolyte ImbalancesDocument50 pagesElectrolyte ImbalancesChris ZantiraNo ratings yet
- Fluid and Electrolyte Imbalance PDFDocument21 pagesFluid and Electrolyte Imbalance PDFShafaq AlamNo ratings yet
- Fluid & Electrolytes Balance NewDocument21 pagesFluid & Electrolytes Balance NewPamela Ria Aguinaldo HensonNo ratings yet
- Physiology 1 Fluid ElectrolyteDocument38 pagesPhysiology 1 Fluid ElectrolyteHaziq KamardinNo ratings yet
- Endocrine Pathophysiology Nursing Notes - Part 2Document10 pagesEndocrine Pathophysiology Nursing Notes - Part 2grad_nurse_2015100% (1)
- Endocrine Pathophysiology Nursing NotesDocument4 pagesEndocrine Pathophysiology Nursing Notesgrad_nurse_2015100% (2)
- Acid Base Balance Pathophysiology NursingDocument7 pagesAcid Base Balance Pathophysiology Nursinggrad_nurse_2015100% (2)
- Guidelines Postpartum AssessmentDocument2 pagesGuidelines Postpartum Assessmentgrad_nurse_2015100% (1)
- Charting GuidelinesDocument4 pagesCharting Guidelinesgrad_nurse_2015No ratings yet
- Cancer Pathophysiology Nursing NotesDocument8 pagesCancer Pathophysiology Nursing Notesgrad_nurse_2015100% (2)
- Pediatric Issues in MobilityDocument5 pagesPediatric Issues in Mobilitygrad_nurse_2015No ratings yet
- Unit 1 NOTESDocument11 pagesUnit 1 NOTESgrad_nurse_2015No ratings yet
- Drugs Affecting BPDocument36 pagesDrugs Affecting BPm1k0eNo ratings yet
- Renal Mini Case StudyDocument7 pagesRenal Mini Case Studyapi-242589113No ratings yet
- Functions of The Urinary System Anatomy of The Kidney Urine FormationDocument71 pagesFunctions of The Urinary System Anatomy of The Kidney Urine FormationmaninagaNo ratings yet
- Cardiovascular Drugs: Dr. April Dawn R. LuceroDocument122 pagesCardiovascular Drugs: Dr. April Dawn R. LuceroRjDNo ratings yet
- Electrolytes: George A. HarwellDocument29 pagesElectrolytes: George A. HarwellWho Knows100% (1)
- Thesis HSF 2011 Moholisa RetsilisitsoeDocument121 pagesThesis HSF 2011 Moholisa RetsilisitsoeProudAfurakanNo ratings yet
- Jurnal Biokimia KalsiumDocument5 pagesJurnal Biokimia KalsiumdimitriprawiraNo ratings yet
- SGLT2Document14 pagesSGLT2Rezultate DMD ElytisNo ratings yet
- Benazepril Synthesis - by DR ANTHONY CRASTODocument46 pagesBenazepril Synthesis - by DR ANTHONY CRASTOAnthony Melvin Crasto Ph.DNo ratings yet
- Disease Deficient Enzyme Cardinal Clinical Features Glycogen Structure Von Gierke'sDocument84 pagesDisease Deficient Enzyme Cardinal Clinical Features Glycogen Structure Von Gierke'sclubstar100% (4)
- By Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4Document4 pagesBy Duy Thai, 1997: Pharmacology Semester 1 Page 1 of 4ravi2likeNo ratings yet
- Domingo, Precious Mae TDocument56 pagesDomingo, Precious Mae Tbevzie datuNo ratings yet
- Fluid and Electrolyte ImbalanceDocument27 pagesFluid and Electrolyte ImbalanceSimmi Sidhu100% (1)
- Path Cvs McqsDocument33 pagesPath Cvs McqsShanzay KhanNo ratings yet
- Body Fluid, MML, 2021Document51 pagesBody Fluid, MML, 2021Boon AimanNo ratings yet
- Ganong WF. Cardiovascular Homeostasis in Health and DiseaseDocument30 pagesGanong WF. Cardiovascular Homeostasis in Health and DiseaseM Adil Ali100% (2)
- Pathophysiology Diagram of Congestive Heart FailureDocument3 pagesPathophysiology Diagram of Congestive Heart Failurea_samiane64% (11)
- AMA Manual of Style GuideDocument5 pagesAMA Manual of Style GuideAbo Ahmed TarekNo ratings yet
- Biochem Final Exam MCQs PDFDocument173 pagesBiochem Final Exam MCQs PDFzeeshan jaskaniNo ratings yet
- Adrenal GlandDocument2 pagesAdrenal GlandAurelia AlexandraNo ratings yet
- Brahmbhatt 2018Document7 pagesBrahmbhatt 2018Jr SparkNo ratings yet
- GOOD ANSWERS 1 Past Paper Answers Sem 1Document77 pagesGOOD ANSWERS 1 Past Paper Answers Sem 1abdulNo ratings yet
- Cardiovascular Physiology Practice QuestionsDocument4 pagesCardiovascular Physiology Practice QuestionsHayley Welsh100% (1)
- 1 Biomedical Sciences Q&ADocument83 pages1 Biomedical Sciences Q&Abhaveshnidhi64100% (4)
- ACE Inhibitors - Blood HypertensionDocument2 pagesACE Inhibitors - Blood HypertensionErik SilvaNo ratings yet
- Medsurg Midterm LecturesDocument33 pagesMedsurg Midterm LecturesLongyapon Sheena StephanieNo ratings yet
- Dent Clin N Am 50 (2006) 547-562Document16 pagesDent Clin N Am 50 (2006) 547-562lalajanNo ratings yet
- Urinary SystemDocument95 pagesUrinary SystemCloud D. LuffyNo ratings yet
- OLISET 20 Brand Plan SummaryDocument41 pagesOLISET 20 Brand Plan SummaryEskag SuvidaNo ratings yet
- Quizlet Test 10 CHP 50,51,52Document7 pagesQuizlet Test 10 CHP 50,51,52Jacqueline GreerNo ratings yet