Professional Documents
Culture Documents
Ovarian Dysgerminomas Pathology
Uploaded by
Utiya Nur LailiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ovarian Dysgerminomas Pathology
Uploaded by
Utiya Nur LailiCopyright:
Available Formats
Ovarian Dysgerminomas Pathology Overview of Ovarian
Dysgerminomas
Author: Florette K Gray Hazard, MD; Chief Editor: Ramya Masand, MD more...
Updated: Oct 20, 2015
Overview of Ovarian Dysgerminomas
Differentiating Ovarian Dysgerminomas
Laboratory Markers
Gross and Microscopic Features
Immunohistochemistry
Molecular and Genetic Features
Prognosis of Ovarian Dysgerminomas
Show All
Multimedia Library
References
Overview of Ovarian Dysgerminomas
A dysgerminoma is a tumor of the ovary that is composed of primitive, undifferentiated germ cells. Germ cell
tumors arise from primordial germ cells of the ovary and the testis; however, pathogenesis of the ovarian germ
cell tumors is unknown. Of the ovarian lesions, 97% are benign proliferations (ie, matureteratomas; the
remaining 3% are malignant.[1]
Germ cell tumors may be distinguished by their line of differentiation. Primitive, unipotential germ cells are the
precursors to ovarian dysgerminomas and their testicular analogue, the seminoma. However, pleuripotential
germ cells diverge along several lines of differentiation: trophoblasts, choriocarcinoma; embryonic cells,
embryonal carcinoma; extraembryonic components (endoderm, mesoderm, ectoderm), teratoma; presomite
embryoid bodies, polyembryoma; and yolk sac, yolk sac (endodermal sinus) tumor.
Dysgerminomas are the most common malignant germ cell tumor occurring in theovary (see the following
image), and these lesions are found most commonly in adolescents and young adults; in fact, approximately
60% of cases are diagnosed in patients younger than 20 years. [2] Common signs and symptoms of ovarian
dysgerminomas include abdominal/pelvic pain (55-85%), abdominal mass (35%), fever (10-25%), vaginal
bleeding (10%), and, occasionally, ascites. Unlike other germ cell tumors, dysgerminomas often occur
bilaterally (approximately 10-20% of cases).
and percentages of malignant germ cell tumors.
Extraovarian tumor spread of dysgerminomas often involves the retroperitoneal and pelvic lymph nodes; these
tumors are highly susceptible to radiotherapy.[1] In addition, hematogenous spread may occur; common sites of
involvement are the lungs, liver, and bone.[2]
Go to Ovarian Cancer and Borderline Ovarian Cancer for complete information on these topics.
Differentiating Ovarian Dysgerminomas
The chief disorders in the differential diagnosis of dysgerminoma are lymphoma/leukemia, yolk sac
tumor, embryonal carcinoma, and Sertoli cell tumor. Large-cell lymphoma typically occurs bilaterally; it is
recognized on positive staining of markers for CD45 (LCA) but not for OCT3/4, PLAP, or SALL4. [3]
Yolk sac tumor may exhibit a solid pattern, simulating dysgerminoma, but other classic architectural patterns of
yolk sac tumor (ie, reticular, microcystic, glandular, or Schiller-Duvall bodies) are also invariably present.
Pure embryonal carcinoma is rare in the ovary. However, mixed germ cell tumors occur in approximately 5% of
cases. When present, embryonal cell carcinoma exhibits more nuclear hyperchromasia and nuclear
pleomorphism, amphophilic cytoplasm, high mitotic index, and necrosis. Often, a glandular or papillary
architecture is present. The cells of embryonal carcinoma express CD30 and cytokeratin (strong, diffuse),
whereas those of dysgerminoma do not.
A study investigated nuclear protein in the testis (NUT) expression in ovarian germ cell tumors (GCTs). The
study found that most malignant ovarian GCTs express NUT protein, albeit focally, and this should be
considered when evaluating immunostaining in the differential diagnosis of poorly differentiated malignancies,
particularly NUT midline carcinoma. The study further advised that since NUT protein appears to play a role in
normal germ cell maturation it may influence intestinal or hepatoid differentiation within malignant GCTs. [4]
Sertoli cell tumors may be mistaken for dysgerminoma when tubules are indistinct and/or solid, especially if
they are poorly fixed. However, Sertoli cell tumors do not usually have a background lymphocytic infiltrate; they
express cytokeratin (strong, diffuse), calretinin, and inhibin.
Laboratory Markers
Dysgerminomas are associated with elevated serum levels of lactate dehydrogenase (LDH).
Although these tumors are thought to be hormonally inert, at least 1 case of precocious puberty occurring in
association with dysgerminoma has been reported.[5]The patient was a 6-year-old girl whose precocious
puberty was caused by elevations in the levels of betahuman chorionic gonadotropin (beta-hCG), alphafetoprotein (AFP), and estradiol.
Additionally, elevated serum levels of neuron-specific enolase, [6] calcium,[7] inhibin,[8]placental alkaline
phosphatase (PLAP), and prolactin [9] have been reported. These serologic elevations readily resolve following
surgical excision; after the elevations resolve, the serum levels may be used as tumor markers to monitor for
recurrence. Because these markers are more commonly associated with other germ cell tumors (ie, yolk sac,
embryonal carcinoma, choriocarcinoma), many scientists contend that secreting dysgerminomas are
misdiagnosed as pure lesions and that they actually represent mixed tumors containing other malignant germ
cell components.[2]
Go to Gynecologic Tumor Markers for complete information on this topic.
Gross and Microscopic Features
Dysgerminomas are characterized by their solid nature and rapid growth. Grossly, these tumors often measure
more than 10 cm in maximum dimension at the time of diagnosis. The classic histology of dysgerminomas
features a proliferation of epithelioid cells admixed with mature lymphocytes arranged in sheets or small
clusters separated by thin, fibrous septae resembling alveoli. The neoplastic cells are large and have moderate
to high nucleus-to-cytoplasm ratios. Other features are squared-off to round nuclei; vesicular chromatin;
prominent nucleoli; clear to eosinophilic cytoplasm rich in glycogen and lipid; and distinct cell borders.
Multinucleated forms may be present. Mitotic activity may be significant and may vary greatly, even within the
same tumor; atypical mitoses may be seen. Noncaseating granulomas, syncytiotrophoblastlike giant cells, and
germinal center formation are not uncommon. Additionally, foci of hemorrhage, necrosis, and small
microcalcifications may also be identified.
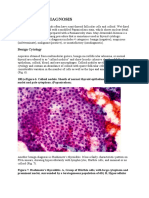
Examples of ovarian dysgerminoma histology are shown below.
Ovarian dysgerminoma histology (hematoxylineosin, 100)
Ovarian dysgerminoma histology. The arrows
indicate syncytiotrophoblastlike giant cells. A and B: Hematoxylin-eosin, 200. C: hematoxylin-eosin, 400. D: hematoxylineosin, 600.
Although numerous architectural variants exist, the cytologic features remain constant. These varying
architectural patterns include but are not limited to sparse lymphocytes, trabeculae, microcysts, and tubules, as
well as extensive hyalinization. As yet, no prognostic significance has been attributed to these architectural
patterns.
Dysgerminomas demonstrate a characteristic tigroid background and loosely cohesive, polygonal cells with
round to oval nuclei, vesicular chromatin, and multiple (1-4) distinct nucleoli on cytology preparations.
Immunohistochemistry
Immunohistochemistry (IHC) plays an important role in characterizing germ cell tumors. Although the wide
range of architectural patterns may make establishing the pathologic diagnosis difficult, the
immunohistochemical profile is often informative.[1, 2]
The neoplastic cells of dysgerminomas express placental alkaline phosphatase (PLAP), CD117 (c-kit), OCT
3/4, SALL4, and, variably, cytokeratin (see the images below). They do not express epithelial membrane
antigen (EMA), S100 protein, CD45 (LCA), or alpha-fetoprotein (AFP).
Dysgerminoma immunohistochemistry (x200).
CD117 = a proto-oncogen (c-kit) ; CKAE1/CAM5.2 = cytokeratins; D2-40 = a monoclonal antibody; H&E = hematoxylineosin; OCT 3/4 = a transcription factor; PLAP = placental alkaline phosphatase.
Syncytiotrophoblastlike giant cells are the source of beta-hCG production; this protein expression is confirmed
by immunohistochemistry. D2-40 membrane expression has been established in testicular seminoma[10] but has
not been extensively explored in dysgerminoma.
Molecular and Genetic Features
Isochromosome 12 (i(12p)) is seen in dysgerminomas, as it is seen in seminomas of the testis. A wide array of
genetic syndromes has been associated with ovarian dysgerminomas; however, many associations are loose,
and establishing molecular/genetic relationships is problematic. These syndromes include but are not limited to
Frasier syndrome,[11] pseudo-Meigs syndrome,[12] ataxia-telangiectasia,[13]and Apert syndrome.[14]
Swyer syndrome is an important disorder of intersex that has been shown to have a close relationship with
dysgerminoma, as well as its in-situ counterpart, gonadoblastoma. [15, 16] Swyer syndrome (pure gonadal
dysgenesis) is a disorder of sexual differentiation that is characterized by mutations of the SRY gene
responsible for male sex characteristics. This gene is located on the Y chromosome (p11.31); mutations at this
site result in phenotypic females with mllerian external genitalia, underdeveloped (streak) internal gonads,
amenorrhea, and rudimentary development of breasts, pubic hair, and axillary hair. These patients are at
increased risk (approximately 20-50% by adulthood) for dysgerminoma/gonadoblastoma in one or both ovaries.
[16]
Gonadoblastomas are neoplastic proliferations found almost exclusively in patients with gonadal dysgenesis
(pure or mixed), male pseudohermaphroditism, and Turner syndrome (45,XO and mosaics). More than 90% of
patients with gonadoblastomas have a Y chromosome.
Gonadoblastomas are histologically characterized by expanded nests containing Sertoli cells, germ cells, and
granulosa cells admixed with variable amounts of hyaline. These nests morphologically resemble the CallExner bodies associated with granulosa cell tumors. Leydig cells may also be found within the interstitium.
Characteristically, these neoplastic nests and/or fibrotic stroma between nests often contain large, irregular
calcifications. Mitotic activity, cytologic atypia, and necrosis are minimal or absent.
The following images depict examples of gonadoblastoma histology.
Dysgerminoma and gonadoblastoma histology.
A: Dysgerminoma with an adjacent zone of necrosis and large calcifications (40). B: Gonadoblastoma with an adjacent
dysgerminoma (40). C: Gonadoblastoma with an adjacent dysgerminoma (40). D: Gonadoblastoma (200).
Gonadoblastoma immunohistochemistry (200).
CD117 = a proto-oncogen (c-kit), ; CKAE1/CAM5.2 = cytokeratins; D2-40 = a monoclonal antibody; H&E = hematoxylineosin; OCT 3/4 = a transcription factor; PLAP = placental alkaline phosphatase.
It is not uncommon to see regions of neoplastic overgrowth by dysgerminoma; however, other malignant germ
cell elements may be seen, such as yolk sac and choriocarcinoma. [1, 2]
Prognosis of Ovarian Dysgerminomas
The prognosis and treatment of dysgerminomas are associated with their pathologic and clinical stage. The
overwhelming majority (approximately 75%) of these tumors are limited to one or both ovaries (International
Federation of Gynecologists and Obstetricians [FIGO] stage 1) at the time of diagnosis.
Patients with stage 1A disease (ie, disease that is limited to 1 ovary) may be treated by unilateral
oophorectomy alone, especially when fertility is to be maintained. The relapse rate ranges from 10% to 20%;
the overall survival rate is 90-100%.[17]Patients who suffer relapses may undergo chemotherapy; the survival
rate for such patients is greater than 90%.
Radiotherapy and 3-4 cycles of adjuvant chemotherapy with cisplatinum, etoposide/vinblastine, and bleomycin
are often reserved for patients with at least stage 1B disease (ie, disease that is limited to both ovaries) or for
those patients who suffer recurrences. For these patients, the results of therapy are similar to those of patients
with stage 1A disease.[18]
You might also like
- Ovarian Dysgerminomas Pathology Overview of Ovarian DysgerminomasDocument7 pagesOvarian Dysgerminomas Pathology Overview of Ovarian DysgerminomasDevi SyamNo ratings yet
- Yolk Sac Tumor EmedicineDocument5 pagesYolk Sac Tumor EmedicineUtiya Nur LailiNo ratings yet
- Ovarian Malignant Germ Cell Tumors PDFDocument26 pagesOvarian Malignant Germ Cell Tumors PDFFernanda RiveraNo ratings yet
- Practicalreviewof Ovariansexcord-Stromal Tumors: Krisztina Z. Hanley,, Marina B. MosunjacDocument34 pagesPracticalreviewof Ovariansexcord-Stromal Tumors: Krisztina Z. Hanley,, Marina B. MosunjacJulia LugonNo ratings yet
- Female Genital System PathologyDocument6 pagesFemale Genital System PathologyHelenCandyNo ratings yet
- Cornejo 2019 Sex Cord Stromal Tumors of The TestDocument10 pagesCornejo 2019 Sex Cord Stromal Tumors of The TestfelipeNo ratings yet
- Extragonadal Germ Cell Tumors: H.-J. SchmollDocument8 pagesExtragonadal Germ Cell Tumors: H.-J. SchmollAshutoshNo ratings yet
- Pathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisDocument11 pagesPathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisLatansa DinaNo ratings yet
- AlsnlasnfDocument13 pagesAlsnlasnfDina A. ŠabićNo ratings yet
- Praktikum Reproduksi 2Document46 pagesPraktikum Reproduksi 2Andika Tatag100% (1)
- Flash Points in Special Pathology (Compiled) by Medical Study CenterDocument55 pagesFlash Points in Special Pathology (Compiled) by Medical Study Centerhhewadamiri143No ratings yet
- Definition of NeoplasiaDocument3 pagesDefinition of NeoplasiaMugiwara YzabelNo ratings yet
- Epithelial Carcinoma of The Ovary, Fallopian Tube, and Peritoneum HistopathologyDocument14 pagesEpithelial Carcinoma of The Ovary, Fallopian Tube, and Peritoneum HistopathologyKerlyn GuerraNo ratings yet
- Pathology of Germ Cell Tumors of The TestisDocument14 pagesPathology of Germ Cell Tumors of The TestisAnonymous be1sWu6l6No ratings yet
- Wilms Tumor NelsonDocument8 pagesWilms Tumor NelsonvegaNo ratings yet
- Stem CellsDocument16 pagesStem CellsdregopokeNo ratings yet
- Neoplasma OvarialDocument9 pagesNeoplasma OvarialVicky TanNo ratings yet
- Immunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsDocument30 pagesImmunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsFarah MutiaraNo ratings yet
- Testicular Tumours 2020Document55 pagesTesticular Tumours 2020Ramesh ReddyNo ratings yet
- Background: Muir-Torre SyndromeDocument11 pagesBackground: Muir-Torre Syndromeadela_97lineNo ratings yet
- Neoplasms of TestisDocument26 pagesNeoplasms of TestisFeddyFebriyantoManurungNo ratings yet
- G Path-NeoplasiaDocument60 pagesG Path-Neoplasiachouchou124No ratings yet
- Introduction To OncologyDocument58 pagesIntroduction To OncologyserviceNo ratings yet
- Germ Cell TumoursDocument32 pagesGerm Cell Tumoursapi-3705046No ratings yet
- Non-Epithelial Ovarian Cancers: Risk ManagementDocument3 pagesNon-Epithelial Ovarian Cancers: Risk ManagementAngga PrabawaNo ratings yet
- Anatomy and Pathology of Testicular Tumors - UpToDateDocument26 pagesAnatomy and Pathology of Testicular Tumors - UpToDateBhargav YagnikNo ratings yet
- Citologie-Fna Tiroida DocumentDocument10 pagesCitologie-Fna Tiroida DocumentMocanu RalucaNo ratings yet
- Overgrowth Syndromes: Andrew C. Edmondson Jennifer M. KalishDocument8 pagesOvergrowth Syndromes: Andrew C. Edmondson Jennifer M. KalishJuan Antonio Herrera LealNo ratings yet
- TESTICULAR CANCER BookletDocument33 pagesTESTICULAR CANCER BookletCheeBrendaNo ratings yet
- Pathologic Quiz Case: Ovarian Mass in A 2-Year-Old Girl Presenting With Pleural EffusionsDocument4 pagesPathologic Quiz Case: Ovarian Mass in A 2-Year-Old Girl Presenting With Pleural EffusionsMateen ShukriNo ratings yet
- Molecular Genetics of Colorectal Cancer - UpToDateDocument35 pagesMolecular Genetics of Colorectal Cancer - UpToDateHartemes RosarioNo ratings yet
- Male Genital System - Pathology (Lect 10-12)Document83 pagesMale Genital System - Pathology (Lect 10-12)Farahh ArshadNo ratings yet
- Cancer CellsDocument4 pagesCancer Cellsxdmhundz999No ratings yet
- 6 Tumors IntroductionDocument101 pages6 Tumors IntroductionReZky MusliminNo ratings yet
- @@@tumor MarkersDocument6 pages@@@tumor Markers918832No ratings yet
- EmperipolesisDocument2 pagesEmperipolesisWilmer C. Geri ParedesNo ratings yet
- Multiple MyelomaDocument15 pagesMultiple MyelomaDinda YusditiraNo ratings yet
- Nonepithelial Ovarian Cancers: ReviewDocument10 pagesNonepithelial Ovarian Cancers: ReviewSaeed HasanNo ratings yet
- Other TypesDocument17 pagesOther TypesJuan Maurencius CastleNo ratings yet
- Ovarian TumoursDocument41 pagesOvarian TumoursVlad RazvanNo ratings yet
- Tumor CancerDocument21 pagesTumor CancerdioncheeNo ratings yet
- Deteksi Kanker OvariDocument11 pagesDeteksi Kanker OvariFenny SestrianiNo ratings yet
- Cystosarcoma PhyllodesDocument3 pagesCystosarcoma PhyllodesrahadianharyantoNo ratings yet
- Neoplasms of Testis NishDocument54 pagesNeoplasms of Testis NishRamesh ReddyNo ratings yet
- Breast CancerDocument56 pagesBreast Cancersarguss14No ratings yet
- 331-Book Chapter-3614-2-10-20210406Document20 pages331-Book Chapter-3614-2-10-20210406Yolla GitamayaNo ratings yet
- Causes of Cervical Cancer: Online Medical BooksDocument4 pagesCauses of Cervical Cancer: Online Medical BooksrochelleNo ratings yet
- 4 Review of LiteratureDocument28 pages4 Review of LiteratureSarika RanaNo ratings yet
- Imagenes Citologicas, Benignas, de MamaDocument19 pagesImagenes Citologicas, Benignas, de MamaPauloRosalesNo ratings yet
- It Does Exist Diagnosis and Management of Thyroid Carc 2023 Surgical PatholDocument12 pagesIt Does Exist Diagnosis and Management of Thyroid Carc 2023 Surgical PatholrubenmacaNo ratings yet
- Slide 15 Diseases of Salivary Glands IIDocument64 pagesSlide 15 Diseases of Salivary Glands IIJustDen09100% (1)
- Medsci 06 00031 PDFDocument113 pagesMedsci 06 00031 PDFCarolina VillalobosNo ratings yet
- Rodent OncologyDocument24 pagesRodent Oncology黃皓No ratings yet
- Ew WikiDocument8 pagesEw WikidesyonkNo ratings yet
- Breast Lesions Pic For BoardDocument179 pagesBreast Lesions Pic For BoardDr-Mohammad Ali-Fayiz Al TamimiNo ratings yet
- Cancer Volume 70 Issue Supplement S4 1992 - Genetic Predisposition To Breast CancerDocument8 pagesCancer Volume 70 Issue Supplement S4 1992 - Genetic Predisposition To Breast CancerApache313No ratings yet
- Ovarian CancerDocument10 pagesOvarian Cancerthaismartins023No ratings yet
- PA 29 Testicular TumorsDocument46 pagesPA 29 Testicular TumorsFangNo ratings yet
- Cytogenetics: Alkitab University - Collage of Medical Techniques Department of Medical Analysis 3 Rd. Stage - A4Document4 pagesCytogenetics: Alkitab University - Collage of Medical Techniques Department of Medical Analysis 3 Rd. Stage - A4Mohammed R.HusseinNo ratings yet
- Cancer Biology, a Study of Cancer Pathogenesis: How to Prevent Cancer and DiseasesFrom EverandCancer Biology, a Study of Cancer Pathogenesis: How to Prevent Cancer and DiseasesNo ratings yet
- Maj Pierce Butler Lists of Enslaved People PDFDocument74 pagesMaj Pierce Butler Lists of Enslaved People PDFBrian Sheffey100% (1)
- 1 s2.0 S1319562X19300968 MainDocument11 pages1 s2.0 S1319562X19300968 MainRobert TriarjunetNo ratings yet
- Best Linear Unbiased Prediction of Genomic Breeding PDFDocument8 pagesBest Linear Unbiased Prediction of Genomic Breeding PDFEdjane FreitasNo ratings yet
- 6 (2016) Sustainable Valorisation of Seafood By-Products - Recovery of CollagenDocument15 pages6 (2016) Sustainable Valorisation of Seafood By-Products - Recovery of CollagenMaría Fernanda Moreno ValdésNo ratings yet
- State Level Common Post Graduate Entrance Tests (CPGET) - 2019Document8 pagesState Level Common Post Graduate Entrance Tests (CPGET) - 2019Telika RamuNo ratings yet
- Spermatogenesis PPTDocument42 pagesSpermatogenesis PPTInsatiable CleeNo ratings yet
- Solid Lipid Nanoparticles (SLN)Document33 pagesSolid Lipid Nanoparticles (SLN)reyviNo ratings yet
- 0001DC012834 1 Yf1Document2 pages0001DC012834 1 Yf1Arun KumarNo ratings yet
- EXP 1 - Wastewater Treatment in Aerobic Batch ReactorDocument10 pagesEXP 1 - Wastewater Treatment in Aerobic Batch ReactorAinin SofiyaNo ratings yet
- How To Write Discussions and Conclusions - PLOSDocument10 pagesHow To Write Discussions and Conclusions - PLOSkarenNo ratings yet
- Si RNADocument29 pagesSi RNALuis MonteroNo ratings yet
- Form InspeksiDocument8 pagesForm Inspeksiichsan syahputraNo ratings yet
- Sts Finals NotesDocument10 pagesSts Finals NotesRossy HidalgoNo ratings yet
- Laboratory Manual of Biopharmaceutics and Pharmacokinetics - 1 PDFDocument167 pagesLaboratory Manual of Biopharmaceutics and Pharmacokinetics - 1 PDFkdk;lkd33% (3)
- Progress in PD-1 - PD-L1 Pathway Inhibitors - From Biomacromolecules To Small MoleculesDocument30 pagesProgress in PD-1 - PD-L1 Pathway Inhibitors - From Biomacromolecules To Small MoleculesMario Andres JuradoNo ratings yet
- Cell Disruption Extracting Target Product: Location of Biological ProductsDocument88 pagesCell Disruption Extracting Target Product: Location of Biological ProductsRoshan jaiswalNo ratings yet
- PROMETRA-Uganda Profile 2010Document7 pagesPROMETRA-Uganda Profile 2010Sekagya YahayaNo ratings yet
- CEPHalometryDocument107 pagesCEPHalometrydisha 146jandialNo ratings yet
- Inhibitory Control Over Action and MemoryDocument11 pagesInhibitory Control Over Action and MemoryMaryam TaqaviNo ratings yet
- History of HLA PDFDocument17 pagesHistory of HLA PDFeseNo ratings yet
- 40 Sleep Hacks: The Geek's Guide To Optimizing SleepDocument50 pages40 Sleep Hacks: The Geek's Guide To Optimizing Sleepjackrulez007No ratings yet
- Homework 1 J23Document8 pagesHomework 1 J23Nada Abu ShweimehNo ratings yet
- Capsicum Annuum L., Commonly Known As Hot Pepper or Chilli Is ADocument11 pagesCapsicum Annuum L., Commonly Known As Hot Pepper or Chilli Is Ajavedsaqi100% (1)
- Guide To Z Scores 1.2Document9 pagesGuide To Z Scores 1.2Marco Vinicio Bazzotti100% (1)
- Determination of Caffeine in Tea SamplesDocument10 pagesDetermination of Caffeine in Tea SamplesMaisha KéimNo ratings yet
- Barriers of Protein and Peptide Drug DeliveryDocument12 pagesBarriers of Protein and Peptide Drug DeliveryAashish chaudhari100% (2)
- The Storage of Tropical Agricultural Products: Agrodok 31Document84 pagesThe Storage of Tropical Agricultural Products: Agrodok 31Seyha L. AgriFoodNo ratings yet
- SOL BIO Anatomical Evidence of Evolution Henry PriceDocument7 pagesSOL BIO Anatomical Evidence of Evolution Henry PriceHenry PriceNo ratings yet
- Behold The Mighty DinosaurDocument65 pagesBehold The Mighty DinosaurLuis Fernandez100% (3)
- 1.0 Perpetuation of LifeDocument29 pages1.0 Perpetuation of LifeSomeone SomethingNo ratings yet