Professional Documents
Culture Documents
An Activity Series of Ions Lab
Uploaded by
ShakilMirzaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
An Activity Series of Ions Lab
Uploaded by
ShakilMirzaCopyright:
Available Formats
An Activity Series of Ions Lab Report Shakil M, Horby L, Shaquile P, Fazal M April 11th 2012 Mr.
Vu Chemistry
Introduction: Many reactions occur in our daily lives to benefit and improve our wellness and surroundings. We know that reactions are classified into many types, they are: synthesis reactions, decomposition reactions, single displacement reactions, and double displacement reactions. Here, in this experiment we will have a look on single displacement reactions which includes reactions between metals and metal ions. A single displacement reaction is a reaction in which an element displaces another element in a compound, producing a new element. This will also include the introduction of the reactivity series in which most reactions will have to be referred back to in order to find out which element displaces which in a compound. The reactivity series states that an element can only displace an element from a compound solution below it, and the farther apart two elements are, the more likely it is that the displacement reaction will occur quickly. Purpose: The purpose of this experiment is to find the trend of reactivity of metal ions. Hypothesis: I predict that the trend of reactivity of metal ions used in this experiment will decrease from top to bottom in a group, and will decrease from left to right across a period. The most reactive to least reactive metal ions that would be used in this experiment are Mg2+, Zn2+, Fe2+, Sn2+, Cu2+. Variables The controlled variable is the variable that does not change throughout a test, and in this experiment it will be the amount of the five different dilute solutions, which is 0.1 mol/L. The controlled variable also includes the mass, and the temperature of the dilute solutions, and the well plate which it would be kept in. The manipulated variable is the variable that is purposely changed, and in this case it
would be the five different metals needed to react with the solutions. The sand paper can be included in this type of variable since it is being changed after we smooth the metal strips. The responding variable is a factor that is measured as result of the manipulated variable, and in this case it would be the chemical reactions between the metal strips and the solution. Equipment and Materials Please refer to page 180 of the Nelson Chemistry 11 University Preparation textbook. Procedure 1. One must first wear chemical safety goggles to protect the eyes, as well as lab apron and gloves 2. Clean materials, and tools being used (such as the well plate) and the surrounding workstations 3. Then gather the five metal strips stated in the equipment and materials stated earlier 4. Use the sand paper to clean the metal strips 5. Divide each metal into 5 equal pieces of the specific metal strip so that one metal is in each solution 6. Fill five spots in the well plate of the same solution by placing five droplets in each spot. Repeat this with the other solutions until you fill 5 spots of the well plate. 7. Place one of each strip of magnesium metal in the five different diluted solutions. Make sure each solution is tested with one metal. 8. Repeat step six with zinc, copper, tin and iron 9. Record a reaction only when a new solid forms. 10. Dispose the contents of the well plate as directed by your teacher 11. Clean your workstation and wash your hands
Observations
Ion Metals
Mg (s) Mg2+ (aq) Zn2+ (aq) Fe2+ (aq) Sn2+ (aq) Black coating appears, and shininess is lost, and becomes bigger (like a sponge) after stirring Fur type spikes appear (like a hairball) Cu2+ (aq) Black coating appears, and shininess is lost
Black coating Black coating Black coating appears, and appears, and appears, and shininess is lost shininess is lost shininess is lost
Zn (s)
No Reaction
No Reaction
Some Discolouration
A black coating
Fe (s)
No Reaction
Little Discolouration
No Reaction
Little discolouration
Some bits are floating on top which have black coating, and bits on the bottom have a brown-reddish coating Brown Discolouration Discolouration, it turns silver
Sn (s) No Reaction Cu (s) No Reaction No Reaction No Reaction
Brown colouring, and settles to the bottom No Reaction
No Reaction Little Discolouration
Analyze and Evaluate a) Variables measured/recorded and/or manipulated in this investigation were the metals, Mg(s), Zn(s), Fe(s), Sn(s), and Cu(s) and the responding variables which are the diluted solutions (MgSO4, ZnSo4, FeSO4, SnCl2, and CuSO4). The type of relationship being tested was the ability for the metal to replace a metal ion in the solution to determine whether or not a single displacement reaction will take place.
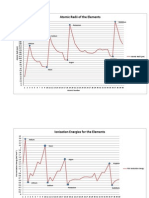
b) The trend of reactivity of metal ions is opposite of the reactivity of metals since based on the activity series, a metal can only displace elements below it from compounds in a solution but cannot displace elements above it. This proves my hypothesis to be incorrect as I had stated earlier that the most stronger reactivity of ions to the least reactivity of ions is Mg2+, Zn2+, Fe2+, Sn2+, Cu2+, when in actuality the trend from most to least reactive is the opposite, Cu2+, Sn2+, Fe2+, Zn2+, Mg2+. In the observation table above, Copper metal reacts with all five metals, which would deduce that it would be the most strongest. Tin solution reacted with four out the five metals, while the iron aqueous reacted with three out of the five metals. The Zinc solution reacted with two metals and the Magnesium aqueous reacted with one solution. c) The trends in reactivity of metals compared to the trends in the reactivity of ions is that reactive metals have unreactive ions such as Mg (s) and Mg2+, and unreactive metals tend to have reactive ions such as Cu (s) and Cu2+. The reactivity of metals follows the same order as the activity series, but the reactivity of metal ions solutions is opposite of the order because the Cu2+ is far more reactive to Mg2+ while comparing the metals, Mg (s) was far more reactive to Cu (s). d) Using the activity series of metals, I predict that the highest to lowest reactive metal ions will be Barium (Ba2+), Nickel (Ni2+), and Mercury (Hg2+). This can be justified by observing the activity series and the trend that metals become most reactive to least reactive from left to right across a row. e) The chemical equations for the reactions in the observations table above is: i) ii) iii) iv) v) Mg + ZnSO4 Fe + ZnSO4 Sn+ ZnSO4 Cu+ ZnSO4 Fe + MgSO4 MgSO4 + Zn FeSO4 + Zn SnSO4 + Zn CuSO4 + Zn FeSO4 + Mg
vi) vii) viii) ix) x) xi) xii) xiii) xiv) xv) xvi) xvii) xviii) xix) xx)
Sn + MgSO4 Cu + MgSO4 Zn+ MgSO4 Mg+FeSO4 Sn+FeSO4 Cu+ FeSO4 Zn+ FeSO4 Mg + SnCl2 Fe + SnCl2 Cu + SnCl2 Zn + SnCl2 Mg+CuSO4 Fe+CuSO4 Sn+CuSO4 Zn+CuSO4
SnSO4+ Mg CuSO4 + Mg ZnSO4+Mg MgSO4 + Fe SnSO4 + Fe CuSO4 + Fe ZnSO4 + Fe MgCl2 + Sn FeCl2 + Sn CuCl2 + Sn ZnCl2 + Sn MgSO4 + Cu FeSO4 + Cu SnSO4 + Cu ZnSO4 + Cu
Apply and Extend Some nuclear power plants use seawater as coolant, choosing the correct type of piping to carry seawater is critical because seawater contains trace amounts of dissolved silver and gold ions. Using my knowledge learnt from this experiment and previous knowledge of the activity series I believe that carrying seawater with copper piping will not be suitable. According to the Nelson Chemistry 11 University Preparation textbook, states the farther apart two elements are, the more likely it is that the displacement reaction will occur quickly. To determine whether or not a reaction between an element and a compound will proceed, look at the relative positions of the two metals in the activity series. If the
higher (more active) metal is the element, the reaction proceeds. If the higher metal is in the compound, no reaction occurs. (p. 165) This proves that if gold and silver ion (which are near the bottom of the activity series) would bond together the single displacement reaction would occur because firstly, the ions are at the bottom meaning they are not able to displace any other element above it. Copper and though would be able to displace a gold or silver ion, and thus causing a reaction but since their relative positions between them are very minimal, the reaction will take a very long time. Since the ions also have NaCl moving around them, it will deteriorate the copper which is mainly used to transfer fresh water and not salt/seawater. Thus a single displacement reaction is likely to occur. Conclusion: In conclusion, we can infer from our experiment that the reactivity of metals follows the same order as the activity series, but the reactivity of metal ions solutions is opposite of the order. Hence, single displacement reaction will take place displacing the metal (lower in the activity series) from the compound. Some sources of error that occurred in this experiment could be the clean metal strips used which may have contained impurities which would definitely affect the final result. Another error that may alter the results was that enough time would not have been given for the reaction to proceed. Lastly, human error can also be involved if two different solutions had contaminated one another causing altered results, or when the spot plate had been drained of a previous solution it was not rinsed properly causing a mixture of two aqueous solutions. My hypothesis was wrong in the end since I had stated the reactivity opposite to the actual results of the metal ions which were, Cu2+, Sn2+, Fe2+, Zn2+, and Mg2+. I had confused the reactivity of the metals to the metal ions, and this small difference was ignored which created the wrong hypothesis mentioned above.
You might also like
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- Africanas Journal Volume 3 No. 2Document102 pagesAfricanas Journal Volume 3 No. 2Gordon-Conwell Theological Seminary100% (2)
- Self-Learning Home Task (SLHT) : Key Drawings and Animation BreakdownsDocument6 pagesSelf-Learning Home Task (SLHT) : Key Drawings and Animation BreakdownsRUFINO MEDICONo ratings yet
- 5.4.3 LabDocument4 pages5.4.3 LabSid MathurNo ratings yet
- Speaking C1Document16 pagesSpeaking C1Luca NituNo ratings yet
- Metal Activity Series Virtual LabDocument5 pagesMetal Activity Series Virtual LabFarhan HabibzaiNo ratings yet
- Single Replacement Reactions LabDocument3 pagesSingle Replacement Reactions Labapi-241225667No ratings yet
- gtg60 Cervicalcerclage PDFDocument21 pagesgtg60 Cervicalcerclage PDFLijoeliyas100% (1)
- Cart On A Ramp Lab ReportDocument6 pagesCart On A Ramp Lab ReportShakilMirza0% (2)
- Report On Transition MetalsDocument4 pagesReport On Transition MetalsAndrea Mendoza100% (2)
- Chemistry Laboratory Report MagnoDocument25 pagesChemistry Laboratory Report MagnoMyrelle Eloise DumalaganNo ratings yet
- An Activity SeriesLab ReportDocument7 pagesAn Activity SeriesLab ReportLance MetsgerNo ratings yet
- Experiment #2 / Unit 4 Single Replacement Reactions: Metals Switching Places in SolutionDocument2 pagesExperiment #2 / Unit 4 Single Replacement Reactions: Metals Switching Places in Solutionapi-368121935No ratings yet
- Activity Series Lab ReportDocument7 pagesActivity Series Lab ReportArmann JohalNo ratings yet
- Reactivitty of Metals-1Document3 pagesReactivitty of Metals-1Ejaz YusuffNo ratings yet
- Experiment 7: Redox Reactions and The Metal Activity Series OutcomesDocument4 pagesExperiment 7: Redox Reactions and The Metal Activity Series OutcomesSafwan m.tNo ratings yet
- Reactivity of Metals Lab: ChemistryDocument2 pagesReactivity of Metals Lab: Chemistryshania lewisNo ratings yet
- Lab #6Document8 pagesLab #6EmmaNo ratings yet
- Reactivity LO's and IGCSE NotesDocument10 pagesReactivity LO's and IGCSE Notesm.sarmadrehanNo ratings yet
- Activity Series Lab (Akey)Document4 pagesActivity Series Lab (Akey)Elah Palaganas100% (1)
- Relative Reactivities of Metals LabDocument4 pagesRelative Reactivities of Metals Labapi-250118165No ratings yet
- 1314 Lab - Single Replacement Lab As Inquiry BasedDocument2 pages1314 Lab - Single Replacement Lab As Inquiry Basedapi-239433858No ratings yet
- CHEM111-Experiment No 2Document5 pagesCHEM111-Experiment No 2ryalphawolfNo ratings yet
- College of Dunaújváros Metallurgy: Report 2Document5 pagesCollege of Dunaújváros Metallurgy: Report 2Luiz Gustavo Gomes NoceraNo ratings yet
- Reactivity Series ExperimentDocument3 pagesReactivity Series ExperimentSourabh DasNo ratings yet
- Single Replacement Reactions LabDocument2 pagesSingle Replacement Reactions Labapi-239477691No ratings yet
- Chem Lab Oxidation ReductionDocument3 pagesChem Lab Oxidation ReductionMayara Halper100% (2)
- Metals and Acids LabwsDocument4 pagesMetals and Acids Labwsapi-296242346No ratings yet
- Buthor, Carla Kaye DG .Lab Report 6.1Document5 pagesButhor, Carla Kaye DG .Lab Report 6.1Carla Kaye ButhorNo ratings yet
- The Metal Reactivity SeriesDocument2 pagesThe Metal Reactivity SeriesJacinta MartinNo ratings yet
- Displacement Reactions-Power PointDocument10 pagesDisplacement Reactions-Power PointMuyatwa LiksNo ratings yet
- Chem181-M15 Expt. No. 5 Final Report - MendozaDocument6 pagesChem181-M15 Expt. No. 5 Final Report - MendozaAdrian MendozaNo ratings yet
- Reactions in Our World Lab ReportDocument5 pagesReactions in Our World Lab ReportAshlen DiCiccoNo ratings yet
- Activity SeriesDocument7 pagesActivity SeriesAhmedSaad647No ratings yet
- Metals Notes 10 3YDocument20 pagesMetals Notes 10 3YconstancewtyuenNo ratings yet
- Untitled Document 3Document4 pagesUntitled Document 3Reliza Amahan SenoNo ratings yet
- Marithonchemper 8 SinglereplacementlabDocument2 pagesMarithonchemper 8 Singlereplacementlabapi-241156470No ratings yet
- Chemistry TaskDocument11 pagesChemistry TaskLogayne AhmedNo ratings yet
- COT 3 PowerpointDocument27 pagesCOT 3 Powerpointshalinee.daydayNo ratings yet
- Lab 10 - Single ReplacementDocument3 pagesLab 10 - Single Replacementapi-239436089No ratings yet
- Chemistry AnswersDocument2 pagesChemistry AnswerspmuthuhaasiniNo ratings yet
- Activity of MetalsDocument8 pagesActivity of MetalsDaniel BerryNo ratings yet
- Activity Series LabDocument4 pagesActivity Series LabjeffoisawesomeNo ratings yet
- Class 10th ChemistryDocument17 pagesClass 10th ChemistryasritakilanNo ratings yet
- 1-Basic of CorrosionDocument41 pages1-Basic of CorrosionMohamed Gawad ARayaNo ratings yet
- ChemistryDocument19 pagesChemistryDevanshu KathrechaNo ratings yet
- Gold BibleDocument6 pagesGold BibleJob MateusNo ratings yet
- Miscon PrecipitationDocument12 pagesMiscon PrecipitationKya JewelleryNo ratings yet
- Chemical Properties of MetalsDocument7 pagesChemical Properties of MetalsDAKSH GREAD DPSN-STDNo ratings yet
- Obervations Lab FinalDocument5 pagesObervations Lab Finalapi-239403297No ratings yet
- Reactivity of Metals Lab Write Up SheetDocument2 pagesReactivity of Metals Lab Write Up SheetJadn BrwnNo ratings yet
- 9.1 Oxidation and Reduction 9.1.1 Definitions: Oxidation and Reduction Take Place Together at The Same Time in The SameDocument22 pages9.1 Oxidation and Reduction 9.1.1 Definitions: Oxidation and Reduction Take Place Together at The Same Time in The SameJaimin SuraniNo ratings yet
- Sophia Science Lab ReactivityofmetalsDocument5 pagesSophia Science Lab Reactivityofmetalsapi-237227791No ratings yet
- Single Replacement LabDocument2 pagesSingle Replacement Labapi-239327773No ratings yet
- Metals: Bonding & Structure Properties Alloys Chemical Reactions Reactivity SeriesDocument23 pagesMetals: Bonding & Structure Properties Alloys Chemical Reactions Reactivity SeriespenguinpowerrrrNo ratings yet
- Lab Report 5Document6 pagesLab Report 5Kedai KasutNo ratings yet
- SinglereplacementrxnlabDocument3 pagesSinglereplacementrxnlabapi-239309345No ratings yet
- 0007190-Fundamentals of Corrosion and Corrosion Control ForDocument25 pages0007190-Fundamentals of Corrosion and Corrosion Control ForPDHLibraryNo ratings yet
- Lab - Single Replacement LabDocument2 pagesLab - Single Replacement Labapi-239404996No ratings yet
- Chapter 3science Solutions Chapter 6 Life ProcessesDocument13 pagesChapter 3science Solutions Chapter 6 Life ProcessessumeshmirashiNo ratings yet
- Oxidation-Reduction Reactions Princess...Document34 pagesOxidation-Reduction Reactions Princess...Warren Mark ManguneNo ratings yet
- Single Replacement Reactions LabDocument2 pagesSingle Replacement Reactions Labapi-239386573No ratings yet
- MR Jamil's Paper 1 Chemistry NotesDocument1 pageMR Jamil's Paper 1 Chemistry NotesDaniyal MehmoodNo ratings yet
- Fundamentals of Corrosion and Their Application To Coil-Coated MetalDocument16 pagesFundamentals of Corrosion and Their Application To Coil-Coated MetalkhurshedlakhoNo ratings yet
- Poem - A Soul's CallingDocument3 pagesPoem - A Soul's CallingShakilMirzaNo ratings yet
- The Catcher in The Rye EssayDocument3 pagesThe Catcher in The Rye EssayShakilMirzaNo ratings yet
- Barney's Version EssayDocument5 pagesBarney's Version EssayShakilMirzaNo ratings yet
- "Rafa" Is: Warfa Nï Wajburnï Warzuqnï - Which Means O Allah, Forgive Me, and Have Mercy OnDocument1 page"Rafa" Is: Warfa Nï Wajburnï Warzuqnï - Which Means O Allah, Forgive Me, and Have Mercy OnShakilMirzaNo ratings yet
- Sermon #4 - Trust of AllahDocument2 pagesSermon #4 - Trust of AllahShakilMirzaNo ratings yet
- The Catcher in The Rye and Romeo and Juliet Comparison EssayDocument4 pagesThe Catcher in The Rye and Romeo and Juliet Comparison EssayShakilMirzaNo ratings yet
- Sermon #3 - ObedienceDocument2 pagesSermon #3 - ObedienceShakilMirzaNo ratings yet
- Sermon #2 - AstagfarDocument3 pagesSermon #2 - AstagfarShakilMirzaNo ratings yet
- From Copper To Copper LabDocument8 pagesFrom Copper To Copper LabShakilMirza100% (9)
- Does Religion Have A Meaningful Place in Peoples' Lives TodayDocument3 pagesDoes Religion Have A Meaningful Place in Peoples' Lives TodayShakilMirzaNo ratings yet
- Sermon #1 - Ill Thinking of OthersDocument2 pagesSermon #1 - Ill Thinking of OthersShakilMirzaNo ratings yet
- Chemical Reactions in Water LabDocument3 pagesChemical Reactions in Water LabShakilMirzaNo ratings yet
- Bacteria LabDocument7 pagesBacteria LabShakilMirzaNo ratings yet
- 12 Angry Men QuestionsDocument2 pages12 Angry Men QuestionsShakilMirza100% (1)
- Atomic Radii V Atomic # GraphDocument2 pagesAtomic Radii V Atomic # GraphShakilMirzaNo ratings yet
- SjshagavDocument6 pagesSjshagavindah ayu lestariNo ratings yet
- 2 - The British Legal SystemDocument4 pages2 - The British Legal SystemSTAN GABRIELA ELENANo ratings yet
- By Nur Fatin Najihah Binti NoruddinDocument7 pagesBy Nur Fatin Najihah Binti NoruddinNajihah NoruddinNo ratings yet
- Simple Past Story 1Document7 pagesSimple Past Story 1Ummi Umarah50% (2)
- Message To St. MatthewDocument3 pagesMessage To St. MatthewAlvin MotillaNo ratings yet
- HotsDocument74 pagesHotsgecko195No ratings yet
- Pakistan's Professor Mafia - Pakistan - DAWNDocument5 pagesPakistan's Professor Mafia - Pakistan - DAWNMuhammad Bilal A. RNo ratings yet
- MNT-Notes Pt. 2Document58 pagesMNT-Notes Pt. 2leemon.mary.alipao8695No ratings yet
- A Palace in TimeDocument6 pagesA Palace in TimeSonkheNo ratings yet
- 1Document13 pages1Victor AntoNo ratings yet
- Pro Angular JS (Apress)Document1 pagePro Angular JS (Apress)Dreamtech PressNo ratings yet
- Cayman Islands National Youth Policy September 2000Document111 pagesCayman Islands National Youth Policy September 2000Kyler GreenwayNo ratings yet
- Hermeneutical Phenomenology and Human Enviroment SystemDocument12 pagesHermeneutical Phenomenology and Human Enviroment SystemAllen Rose Buenaflor BuenoNo ratings yet
- Diexis in Red by Taylor SwiftDocument11 pagesDiexis in Red by Taylor SwiftNirmana ArtstikaNo ratings yet
- Chapter 1 (Research)Document6 pagesChapter 1 (Research)Salome CarpioNo ratings yet
- PDFDocument50 pagesPDFWalaa RaslanNo ratings yet
- Accounting For Employee Stock OptionsDocument22 pagesAccounting For Employee Stock OptionsQuant TradingNo ratings yet
- Glgq1g10 Sci Las Set 4 ColoredDocument4 pagesGlgq1g10 Sci Las Set 4 ColoredPogi AkoNo ratings yet
- Ethiopia FormularyDocument543 pagesEthiopia Formularyabrham100% (1)
- Ingles Semana 11. P6. 2Q. 4egb. A y BDocument2 pagesIngles Semana 11. P6. 2Q. 4egb. A y BWendisilla BelenchisNo ratings yet
- Mahabharata Reader Volume 1 - 20062023 - Free SampleDocument107 pagesMahabharata Reader Volume 1 - 20062023 - Free SampleDileep GautamNo ratings yet
- Songs of KabirDocument342 pagesSongs of KabirSant MatNo ratings yet
- Research in NursingDocument54 pagesResearch in Nursingrockycamaligan2356No ratings yet
- Sepulveda v. de Las CasasDocument2 pagesSepulveda v. de Las CasasNova GaveNo ratings yet
- Last Speech of Shri Raghavendra SwamyDocument5 pagesLast Speech of Shri Raghavendra SwamyRavindran RaghavanNo ratings yet
- Ebook PDF The Irony of Democracy An Uncommon Introduction To American Politics 17th Edition PDFDocument42 pagesEbook PDF The Irony of Democracy An Uncommon Introduction To American Politics 17th Edition PDFscott.stokley449100% (39)