Professional Documents
Culture Documents
Iron - Carbon System
Uploaded by
Yavana KeerthiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Iron - Carbon System
Uploaded by
Yavana KeerthiCopyright:
Available Formats
IRON CARBON SYSTEM Alloys of the Iron-carbon system include steel and cast iron.
. Alloys with a carbon content up to 2% are known as Steels whereas those having carbon above 2% are called Cast-Irons. Alloys of iron-carbon system are ofthe most vital importance to modern industry due to their extensive, versatile applications The Iron-carbon system provides the most prominent example of heat treatment arid property alteration based on polymorphic transformation and eutectoid decomposition. Because of its outstanding commercial importance, the Iron-carbon system has been studied in more detail than most alloy systems. The primary constituent of Iron-carbon system is the metal Iron. IRON, ALLOTROPY Iron is a relatively soft and ductile metal. Iron has a melting point of 1539C. Iron is allotropic metal, which means that it exists in more than one type of lattice structure (e.g., B.C.C./F.C.C.) depending upon temperature. In its normal room temperature state, iron is B.C.C. in lattice arrangement, whereas at 908C it changes to F.C.C. and then at 1403C back to B.C.C. again and vice versa. One another change occurs at about 770C (called the Curie point) at which the room temperature magnetic properties of iron disappear and it becomes non-magnetic. The iron remains non-magnetic until the temperature drops back below the Curie point upon which its magnetic properties reappear. Fig. 40.1 shows a cooling curve for pure iron with allotropic forms of iron marked over it. Iron is molten above 1539C. It solidifies in the B.C.C. delta form.
On further cooling at 1400C, a phase change occurs and the atoms rearrange themselves into the (Gamma) form which is F.C.C. and nonmagnetic. On still further cooling at 910C, another phase change occurs from F.C.C. non-magnetic y iron to B.C.C. non-magnetic alpha iron. Finally at 768C, the alpha-iron (B.C.C.) becomes magnetic without a change in lattice structure. MICRO CONSTITUENTS OF IRON AND STEEL When steel is heated above the austenitic temperature (Refer Fig. 40.4) and is allowed to cool under different conditions (e.g., different rates of cooling), the austenite in steel transforms into a variety of microconstituents discussed below. The study of these microconstituents is essential in order to understand Fe-C equilibrium diagram and T.T.T. diagrams. Various micro-constituents are: (a) Austenite (b) Ferrite (c) Cementite (d) Ledeburite (e) Pearlite (f) Bainite
(g) Martensite (h) Troostite (i) Sorbite (a) Austenite: Austenite is the solid solution of carbon and/or other alloying elements (e.g., Mn, Ni, etc.) in gamma iron Carbon is in interstitial solid solution whereas Mn, Ni, Cr, etc., are in substitutional solid solution with iron. Austenite can dissolve maximum 2% carbon at 2066F, the left-hand corner of Fig. 40.4. Austenite has: * * * Tensile strength 10500 kg/cm2. Elongation 10% in 50 mm. Hardness Rockwell C 40 (Approx).
Austenite is normally not stable at room temperature. Under certain conditions, however, it is possible to obtain austenite at room temperature (as in austenite stainless steels). Fig. 40.2 (a) shows microstructure of austenite at room temperature. Austenite is non-magnetic and soft.
(b) Ferrite [Fig. 40.2 (b)] Ferrite is B.C.C. iron phase with very limited solubility for carbon. The maximum solubility is 0.025% carbon at 1333F at extreme left hand corner of Fig. 40.4, and it (i.e., ferrite) dissolves only 0.008% carbon at room temperature. Ferrite is the softest structure that appears on the Fe-C equilibrium diagram. Ferrite has:
* * * (c)
Tensile strength 2800 kg/cm2 (Approx.) Elongation 40% in 50 mm Hardness less than Rockwell C 0 or Rockwell B 90. Cementite Cementite or iron carbide, chemical formula Fe3C, contains 6.67% carbon by
weight.
It is a typical hard and brittle interstitial compound of low tensile strength (approx. 350 kg/cm2) but high compressive strength.
Cementite is the hardest structure that appears on the iron-carbon equilibrium diagram. Its crystal structure is orthorhombic. (d) Ledeburite.
Ledeburite is the eutectic mixture of austenite and cementite. It contains 4.3% carbon. It is formed at about 1130C (2065F). (e) Pearlite (Fig. 40.5) The_pearlite microconstituent consists of alternate lamellae of ferrite and cementite.
Pearlite is the product of austenite decomposition by an eutectoid reaction. Thus, pearlite is an eutectoid mixture containing about 0.8% carbon and is formed at 13330F(7230C), point C in Fig. 40.4 Pearlite is shown in Fig. 40.5; the white ferrite back-ground or matrix which makes up most of the eutectoid mixture contains thin plates of cementite (black). * * Pearlite has Elongation 20% in 50 mm. Hardness Rockwell C 2O.
(f) Bainite (Fig. 40.12) Bainite is the constituent produced in a steel when austenite transforms at a temperature below that at which pearlite is produced and above that at which martensite is formed. Bainite is produced byAustempering Thus bainite is a decomposition product of austenite, consisting of an aggregate of ferrite and carbide. Bainite forms on isothermal transformation at temperatures below the nose of TTT diagram (Refer Fig. 40.10). Bainite is an isothermal transformation product and cannot be produced by continuous cooling. If bainite is formed in the upper part of the temperature range, its appearance is feathery and it is called Feathery Bainite [Fig. 40.12 (a)]; it is known as Acicular Bainite [Fig. 40.12 (b)] if it is formed in the lower part of the temperature range. Acicular bainite resembles tempered martensite because it has somewhat needle like structure.
(g)
Martensite (Fig. 40.13)
Martensite is a metastable phase of steel, formed by transformation of austenite below the Ms temperature (refer Fig. 40.10). Martensite is an interstitial supersaturated solid solution of carbon in alpha-iron and has a bodycentered-tetragonal lattice. Martensite is considered to be highly stressed a-iron which is supersaturated with carbon. Martensite forms as a result of shear-type transformation with virtually no diffusion. Martensite, normally, is a product of quenching. Martensite possesses an acicular or needle-like structure, Fig. 40.13. (h) Troostite
Troostite (Nodular) is a mixture of radial lamellae of ferrite and cementite and therefore differs from pearlite only in the degree of fineness and carbon content which is the same as that in the austenite from which it is formed [Fig. 40.3 (a)].
In steel heat treatment, the troostite, i.e., the microstructure, consisting of ferrite and finely divided cementite is produced on tempering martensite below approximately 450C. The constituent also known as troostite pearlite is produced by the decomposition of austenite when cooled at a rate slower than that which will yield a martensitic structure and faster than that which will produce a sorbitic structure.
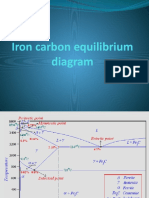
(i) Sorbite [Fig. 40.3 (b)] Sorbite is the microstructure consisting of ferrite and finely divided cementite, produced on tempering martensite/above approximately 450C. The constituent also known as Sorbitic Pearlite, is produced by the decomposition of austenite when cooled at a rate slower than that which will yield a troostitic structure and faster than that which will produce a pearlitic structure. Difference between Pearlite, Sorbite and Troostite. Pearlite, sorbite and troostite are all ferritecementite mixtures having a lamellar structure and distinguishable from each other in eutectoid steel only by their degrees of dispersion. The lower the decomposition temperature (higher degree of super cooling), the more dispersed the ferrite-cementite mixture will be. Pearlite is obtained at low degrees of supercooling. Sorbite, a finer mixture, is obtained at higher degrees of supercooling. At subcritical temperatures in the region of 500 to 550C, troostite, an even more dispersed mixture, is obtained. Under an optical microscope, troostite is observed as a dark mass because the ferrite and cementite particles cannot be resolved. Thus, unlike pearlite, troostite is difficult to differentiate. However, structure of troostite is sufficiently clearly revealed under an electron microscope. IRON CARBON EQUILIBRIUM DIAGRAM An equilibrium, phase or constitutional diagram is a graphic representation of the effects of temperature and composition upon the phases present in an alloy. An equilibrium diagram is constructed by plotting temperature along they-axis and percentage composition of the alloy along the x-axis. This diagram shows ranges of temperatures and compositions within which the various phase changes are stable and also the boundaries at which the phase changes occur. Iron-carbon equilibrium diagram (refer Fig. 40.4) indicates the phase changes that occur during heating and cooling and the nature and amount of the structural components that exist at any temperature. Besides, it establishes a correlation between the microstructure and properties of steel and cast irons and provides a basis for the understanding of the principles of heattreatment. An iron-carbon equilibrium diagram forms a basis for differentiating among iron (0.008% C or less), hypoeutectoid steels (0.008 to 0.8%C), hypereutectoid steels (0.8 to 2.0% C), hypoeutectic cast irons (2 to 4.3% C) and hypereutectic cast irons (above 4.3% carbon). The iron carbon equilibrium diagram has a peritectic (point J) an eutectic (point C) and an eutectoid (point S).
Peritectic reaction equation may be written as ()delta + liquid cooling heating Austenite
The horizontal line at 2720F shows the peritectic reaction. liquid The eutectic reaction takes place at 2066F and its equation may be written as cooling heating austenite +cementite(eutectic mixture{ledeburite})
Eutectic point is at 4.3% carbon. Eutectic mixture is not usually seen in the microstructure, because austenite is not stable at room temperature and must undergo another reaction during cooling. The eitlectaid. reaction is represented by the horizontal line of 1333F and (point) S marks the eutectoid point. The eutectoid equation may be written as solid cooling heating Ferrite + cementite(eutectoid mixture{pearlite})
Transformation which takes place in the structures of steels containing 0.4%, 0.83% and 1.2% carbon respectively (refer Fig. 40.4) when heated to a temperature high enough to make them austenitic and then allowed to cool slowly (under equilibrium conditions), have been explained below. (0 Steel containing 0.4% carbon is a hypoeutectoid steel and is completely austenite above j43, i.e., upper critical temperature line. As it is cooled below A3 line the iron begins to change from F.C.C. to B.C.C. As a result, small crystals of body centered cubic (B.C.C.) iron begin to separate out from the austenite (F.C.C). The B.C.C. crystals retain a small amount of carbon (less than 0.03%) and are referred as crystals of ferrite. As the cooling proceeds, ferrite crystals grow in size at expense of austenite By the time the steel has reached Ax line, i.e., 1333F (called lower critical temperature) it is composed of approximately half ferrite and half austenite. At this stage the austenite contains 0.83% carbon and since austenite can hold no more than 0.83% carbon in solid solution at 1333F (or 723C) thus as the temperature drops further, carbon begins to precipitate as cementite. This cementite and still separating ferrite form alternate layers until all the remaining austenite is consumed. The lamellae structure, i.e., eutectoid of ferrite and cementite contains 0.83% carbon and is known as Pearlite (refer Fig. 40.5). All hypoeutectoid steels when cooled from austenite state will transform into ferrite and pearlite in the same way as explained above
(ii) Consider the transformation of an eutectoid steel containing 0.83% carbon. It will remain austenite up to the point S. The transformation will begin and end at the same temperature, i.e., 1333F (or 723C). Since eutectoid steel contains 0.83% carbon initially, it follows that the final transformed structure will be completely pearlite (see Fig. 40.5). For details refer section 40.7. (iii) Consider the transformation of a hypereutectoid steel (say containing 1.2% carbon). As the temperature drops and steel crossesv4cm (i.e., upper critical temperature) line at point d and moves towards e, the excess carbon above the amount required to saturate austenite (i.e. 0.83%) is precipitated as cementite primarily along the grain boundaries (Fig. 40.4). Thus above 1333F, i.e., lower critical temperature line, the structure consists of austenite and cementite. As the temperature drops below 1333F, the austenite has become less rich in carbon (because of cementite precipitation), it contains only 0.83% carbon and it transforms to pearlite as it does so in the cases of hypoeutectoid and eutectoid steels explained earlier. The structure of a hypereutectoid steel at room temperature consists of cementite and pearlite (Fig. 40.4). So far the discussions were only with regard to structures produced in steels by slow cooling from austenite under equilibrium conditions. In normal foundry practice, the rate of cooling is slightly faster and as a result more cementite plates are nucleated and individual lamellae of pearlite become thinner and the structure is called fine pearlite. If castings are cooled at still faster rate to prevent transformation of austenite above (approximately) 600F, martensite forms on further continuous cooling. Martensite is a hard, strong and brittle constituent. It is a super-saturated solution of carbon in ferrite and the presence of excess carbon distorts the normally cubic ferrite to a body-centered tetragonal structure which is produced by a shear mechanism and is strained. Transformations which take place in the structure of a cast iron containing 3% carbon is shown in Fig. 40.4 and explained as under:
Case (a): Cast iron containing 3% carbon is when cooled under rapid rate as a thin section of a sand casting, from a temperature of about 2500?F, it begins to solidify with the formation of grains of austenite. Austenite continues to solidify until the cast iron reaches the temperature of 2066F. At this stage the alloy consists of 50% austenite and 50% liquid of eutectic composition (austenite and cementite, i.e., ledeburite). As the alloy cools below solidus, i.e., 2066F, ledeburite (a form of eutectic consisting of spheres of austenite embedded in cementite) freezes and cementite precipitates from austenite because of the decreasing solubility of carbon in the austenite. This occurs between 2066 and 1333F. Cooling of the alloy below 1333F* involves the transformation of remaining austenite of eutectoid composition (i.e., 0.83% C) to pearlite as explained earlier for steels. Thus, the structure of alloy at room temperature consists of cementite, pearlite and transformed ledeburite. Cast iron of any composition between 2.0 to 4.3% carbon will solidify in exactly the same way as has been described above. Case (b): If the above very cast iron is cooled at a slow rate, as usual, austenite will first form from the melt (i.e., liquid) but eutectic freezing being slow, products of eutectic reaction will be austenite and graphite [Fig. 40.6 (a)]. This is between 2066 and 1333F. As cooling continues, austenite gets depleted in carbon content and graphite flakes grow. At 1333F, remaining austenite transforms to pearlite and the structure of the alloy at room temperature looks as shown in Fig. 40.6 (b). It is pearlitic gray cast iron.
Case (c): Phase changes in the same alloy when cooled at a very slow rate will be similar to case (b) above, except that, at the eutectoid, (i.e., 1333F) cooling will be sufficiently slow to permit graphite to precipitate rather than pearlite. Although no new graphite flakes^form, the one present, grow in size. Instead of pearlite as in case (b), the matrix of the alloy solidified in this case is ferrite and graphite flakes are embedded in it
EFFECT OF ALLOYING ELEMENTS ON FOUNDING AND OTHER PROPERTIES OF STEELS An alloying element is one which is added to a metal to effect changes in properties and which remains within the metal. Common alloying elements which are added to steel for the purpose are C, Ni, Mo,V W, Mn, Cu, Bo, Al, Co, Si, Ti, Cr, etc. Carbon: Carbon content in steel affects Hardness Tensile strength Machinability Melting point (refer Fig. 40.4) Nickel: Nickel Increases toughness and resistance to impact Lessens distortion in quenching Lowers the critical temperatures of steel and widens the range of successful heat treatment Strengthens steels Renders high-chromium iron alloys austenitic Does not unite with carbon. Chromium: Chromium Joins with carbon to form chromium carbide, thus adds to depth hardenability with improved resistance to abrasion and wear Helps preventing corrosion and oxidation Adds some strength at high temperatures. Molybdenum: Molybdenum Promotes hardenability of steel Makes steel fine grained
Makes steel unusually tough at various hardness levels Counteracts tendency towards temper brittleness Raises tensile and creep strength at high temperatures Enhances corrosion resistance in stainless steels Forms abrasion resisting particles. Vanadium: Vanadium Promotes fine grains in steel Increases hardenability (when dissolved) Imparts strength and toughness to heat-treated steel. Resists tempering and causes marked secondary hardening. Tungsten: Tungsten Increases hardness (and also red-hardness) Promotes fine grain Resists heat Promotes strength at elevated temperatures. Manganese: Manganese Contributes markedly to strength and hardness (but to a lesser degree than carbon) Counteracts brittleness from sulphur Lowers both ductility and weldability if it is present in high percentage with high carbon content in steel. Copper: Copper (0.2 to 0.5%) added to steel increases resistance to atmospheric corrosion Acts as a strengthening agent. Boron: Boron Increases hardenability or depth to which steel will harden when quenched.
Aluminium: Aluminium Acts as a deoxidizer Produces fine austenite grain size If present in an amount of about 1%, it helps promoting nitriding. Cobalt: Cobalt Contributes to red-hardness by hardening ferrite. Silicon: Silicon Improves oxidation resistance Strengthens low alloy steels Acts as a deoxidizer. Titanium: Titanium Prevents localized depletion of chromium in stainless steels during long heating Prevents formation of austenite in high chromium steels Reduces martensitic hardness and hardenability in medium chromium steels. EFFECT OF ALLOYING ELEMENTS ON FOUNDING AND OTHER PROPERTIES OF CAST-IRON Alloying elements are added to cast iron for attaining special properties such as resistance to corrosion, heat and wear and to improve mechanical properties. Many alloying elements in cast iron will accelerate or retard graphitization and this is one of the important reasons for alloying. The most common alloying elements in cast iron are, Cr, Cu, Mo, Ni, V, Mg, Mn and Zr. Chromium: Chromium (0.15 to 1.0%) is used for Hardness Chilling power, i.e., depth of chill Improvement of wear resistance Strength
Resistance to heat. Chromium reduces and refines the graphite. However, chromium decreases machinability. Copper: Copper (0.5 to 2.0%) Toughens the matrix Increases the fluidity Tends to break up massive cementite and strengthens matrix Assists in control of chill depth Tends to increase and refine the graphite. Molybdenum: In conjunction with Ni, Cu and Cr, Mo is used to produce high strength cast irons. Molybdenum (0.3 to 1.0%) Improves mechanical properties such as tensile strength, fatigue strength and hardness. Refines the graphite and pearlite Is a mild stabilizer of carbides Improves heat resistance Retards the transformation of austenite and thus increases hardenability and freedom from cracking and distortion. Vanadium: Vanadium (0.15 to 0.5%) Is a powerful carbide former Stabilizes cementite and improves the structure of the chill Reduces graphitization Improves tensile strength, transverse strength and hardness Adds resistance to wear and heat. Nickel: Nickel (0.1 to 3.0%) Stabilizes austenite Refines pearlite and graphite
Is a mild graphitizer Improves toughness and density of castings Minimizes extremes of hardness between light and heavy sections. Magnesium: Magnesium (0.04 to 0.08%) Produces graphite in spheroidal form in as cast alloys Increases ductility. Manganese: Manganese (0.3 to 1.25%) Stabilizes austenite Refines the graphite and pearlite Acts as a deoxidizer Refines grains Increases fluidity and density in castings. Zirconium: Zirconium (0.10 to 0.3%) Assists formation of graphite Deoxidizes and improves the fluidity and density of castings Reduces hardness.
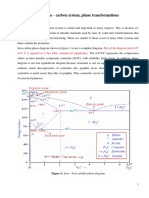
TTT DIAGRAM 1. Introduction T.T.T. (Time-Temperature-Transformation) diagram is also known as S-Curve, C-Curve, Bains Curve or Isothermal Transformation diagram. A T.T.T. diagram shows the relationship between temperature and time (Fig. 40.12) taken for a decomposition transformation to take place in a metal when the transformation is isothermal, i.e., transformation is allowed to occur at constant temperature. T.T.T. diagram is used more particularly in the assessment of decomposition of austenite in a heat treatable steel. 2 Difference from Fe-C Equilibrium Diagram (and Importance of TTT Diagram)
Many heat treatments of steels involve reaction conditions so far removed from equilibrium that Fe-C equilibrium diagram is of only limited use in the study of steels cooled under nonequilibrium conditions. The iron-carbon equilibrium diagram shows only the phases and the resulting microstructures corresponding to equilibrium conditions. The usefulness of Fe-C diagram is restricted to fixing the austenitiz-ing temperature and predicting the phases that are eventually obtained at a given composition (C%age) and temperature. Many metallurgists realized that time and temperature of austenite transformation had a profound influence on the transformation products and the subsequent properties of the steel. For example, the microstructure and properties of a (quenched) steel are dependent upon the rate of cooling which prevails during quenching. As the cooling rate increases, the experimentally observed transformation temperatures are lowered and metastable (non-equilibrium) phases may be formed. For example, at very high rates of cooling in the steel, a metastable phase called martensite can develop which, of course, has no place in the Fe-C equilibrium diagram. The principal source of information on the actual process of austenite decomposition under non-equilibrium conditions is the TTT Diagram, which relates the transformation of austenite to the time and temperature conditions to which it is subjected. 3 Steps to construct a T.T.T diagram 1.Obtain a large number of relatively small specimens (cut from the same bar). 2.Place the samples in a molten salt bath held at the proper austenitiz-ng temperature, [refer Fig. 40.11 (a)]. For a 1080 (eutectoid) steel, this temperature is approximately 1425F.
Specimens are kept in the molten salt bath for long enough to form complete austenite. 3.When austenitized, the samples are quickly transferred to an other molten salt bath held at the desired reaction temperature below A1, for example at 1300F. 4.After a given specimen has been allowed to react isothermally for a certain time, it is quenched in cold water or iced brine. The first specimen may be allowed to react isothermally for 2 sees., second specimen for 4 sees., third for 8 sees., fourth for 15 sees., and so on up to say 15 hours (Fig. 40.11). The more the time is given to a specimen to react isothermally the more pearlite is formed [Fig. 40.11 (&)]. 5. As the specimen is quenched in water, this stops the isothermal reaction (or heat treatment) by causing the remaining (untransformed) austenite to change almost instantly to martensite. In microstructures shown in Fig. 40.11 (b), both pearlite and martensite (white portion) can be seen. Pearlite is the result of isothermal heat treatment and its amount depends upon the time permitted for isothermal reaction to continue. Martensite is the result of water quenching of the specimen after the isothermal heat treatment. 6.When a large number of specimens isothermally reacted for varying time periods are metallographically examined, the result is the Reaction Curve as shown in Fig. 40.11 (b). 7.When the data obtained from a series of isothermal reaction curves over the whole temperature range of austenite instability for a given composition of steel is summarized, the result is TTT diagram for that steel. Fig. 40.12 shows the TTT diagram for an eutectoid (about 0.8%) carbon steel. Austenite is stable above Ax temperature line, and below this line, austenite is unstable, i.e., it can transform into pearlite, bainite or martensite. In addition to the variations in the rate of transformation with temperature, there are variations in the structure of the transformation products also. Transformations at temperatures between approximately 1300F and 1020F (550C) result in the characteristics lamellar micro-structure of pearlite. At a temperature just belowAx line, nucleation of cementite from austenite will be very slow, but diffusion and growth of nuclei will proceed at maximum speed, so that there will be few large lamellae and the pearlite will be coarse.
However, as the transformation temperature is lowered i.e., it is just above the nose of the Ccurve, the pearlite becomes fine. At temperatures between 1020F and 465F (the approximate, Ms temperature line), transformation becomes more sluggish as the temperature falls, for, although austenite becomes increasingly unstable, the slower rate of diffusion of carbon atoms in austenite at lower temperatures outstrips the increased urge of the austenite to transform. In this temperature range the transformation product is bainite. Bainite consists, (like pearlite) of a ferrite matrix in which particles of cementite are embedded. The individual particles are much finer than in pearlite. The appearance of bainite may vary between feathery- mass [Fig. 40.16 (c)] of fine cementite and ferrite for bainite formed around 900F; and dark acicular (needle-shaped) crystals [Fig. 40.16 (d)] for bainite formed in the region of around 600F. - At the foot of the TTT diagram, there are two lines Ms (240C or 465F) andM/(-50C). Ms represents the temperature at which the formation of martensite will start and Mf, the temperature at which the formation of martensite will finish during cooling of austenite through this range. Mf k a fairly low temperature. Martensite is formed by the diffusionless transformation of austenite on rapid cooling to a temperature below 465F (approximately) designated as Ms temperature. The martensitic transformation differs from the other transformations in that it is not time dependent and occurs almost instantaneously, the proportion of austenite transformed to martensite depends only on the temperature to which it is cooled. For example the approximate temperatures at which 50% and 90% of the total austenite will, on quenching, transforms to martensite are 330F and 240F respectively. Fig. 40.13 shows the effect of cooling rate on the formation of different reaction products e.g., pearlite, bainite and martensite
Cooling Curve-a: Very slow cooling rate, typical of conventional annealing. Transformation product is coarse pearlite with low hardness. Cooling Curve-b: Transformation will start at 3 with the formation of coarse pearlite and finish at 4, with the formation of medium pearlite. Since there is a greater temperature difference between point 3 and 4 than there is between 1 and 2, the structure will show a greater variation in the fineness of pearlite and a smaller proportion of coarse pearlite as compared to that of curve-a. Curve-b involves a faster cooling rate than curve a (annealing) and may be considered typical of normalizing. Cooling Curve-c: This curve is typical of a slow oil quench and the microstructure will be a mixture of medium and fine pearlite. Cooling Curves-d: This curve is typical of an intermediate cooling rate and austenite will start to transform (at point 5) to fine pearlite. As Ms line is crossed, the remaining austenite will transform to martensite. The final structure at room temperature will thus consist of martensite and fine pearlite. Cooling Curve-e: This curve is typical of a drastic quench, the substance remains austenitic until the Ms line is reached, and changes to martensite between the Ms and My lines. Cooling Curve-ef: It is possible to form 100% pearlite or 100% martensite by continuous cooling, but it is not possible to form 100% Bainite. Cooling Curve-ef obtains a bainitic structure by cooling rapidly enough to miss the nose of curve and then holding in the temperature range at which bainite is formed until transformation is complete.
Cooling Curve-g: This curve is tangent to the nose of TTT curve. The cooling rate associated with curve-g is approximate critical cooling rate (CCR) for this steel. Any cooling rate equal to or faster than CCR (e.g., cooling rate-e) will form only martensite and any cooling rate slower than CCR (e.g., cooling rates a, b and c) will form some softer transformation products such as pearlite or bainite.
You might also like
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- The IronCarbide DiagramDocument11 pagesThe IronCarbide DiagramshajjikhalidNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Ironiron CarbideequilibriumphasediagramDocument39 pagesIroniron CarbideequilibriumphasediagramSheikh UMARNo ratings yet
- Iron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018Document30 pagesIron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018prasenjitsayantan100% (1)
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- Lesson 5 - Fe-C Diagram - Rev. 0Document11 pagesLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- Engineering Material II Short NoteDocument17 pagesEngineering Material II Short NotewondimuNo ratings yet
- Introduction-Iron Carbon Phase DiagramDocument31 pagesIntroduction-Iron Carbon Phase DiagramTHE BBEASTNo ratings yet
- Heat Treatment of SteelDocument51 pagesHeat Treatment of SteelRAMA BAGAS ADITYA TM 2DNo ratings yet
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianNo ratings yet
- Iron-Carbon Phase DiagramDocument30 pagesIron-Carbon Phase Diagramjunaid hassanNo ratings yet
- Iron-Carbide Phase Diagram AnalysisDocument26 pagesIron-Carbide Phase Diagram AnalysisHiral HiraniNo ratings yet
- Practical 1Document9 pagesPractical 1Sami Onur VuralNo ratings yet
- Metallury of SteelsDocument10 pagesMetallury of SteelsDalitso MwanzaNo ratings yet
- Engineering Materials 27-29Document40 pagesEngineering Materials 27-29Sanu SouravNo ratings yet
- M. Tech. (FFT) Technology of Ferrous Casting Phase DiagramDocument7 pagesM. Tech. (FFT) Technology of Ferrous Casting Phase DiagramRajulapati Sunil KumarNo ratings yet
- Metastable Iron-Carbon (Fe-C) Phase DiagramDocument3 pagesMetastable Iron-Carbon (Fe-C) Phase DiagramupenderNo ratings yet
- TEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ADocument5 pagesTEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ARhea GaiaNo ratings yet
- Plain Iron Carbon SteelsDocument5 pagesPlain Iron Carbon Steelsروشان فاطمة روشانNo ratings yet
- What Is PearliteDocument4 pagesWhat Is Pearliteardy cornettoNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 pagesEngineering Material-II: Iron Carbide Phase DiagramAla ZiNo ratings yet
- Heat Treatment: Dr. Santosh S. HosmaniDocument7 pagesHeat Treatment: Dr. Santosh S. Hosmaniprakush01975225403No ratings yet
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Fe-C Equilibrium Diagram PhasesDocument2 pagesFe-C Equilibrium Diagram Phasesروشان فاطمة روشانNo ratings yet
- Ferrous Alloys Phase Diagram and MicrostructuresDocument7 pagesFerrous Alloys Phase Diagram and MicrostructuresKeshav DesaiNo ratings yet
- The Microstructural Nature of Carbon Steels Phase DiagramDocument4 pagesThe Microstructural Nature of Carbon Steels Phase Diagramamitkharb111195No ratings yet
- Heat Treatment of Metals and AlloysDocument8 pagesHeat Treatment of Metals and AlloysBalveer CLNo ratings yet
- Iron Carbide Phase Diagram AnalysisDocument35 pagesIron Carbide Phase Diagram AnalysisbotobotoakbarNo ratings yet
- IRON CARBON Equilibrium Diagram BME 3Document4 pagesIRON CARBON Equilibrium Diagram BME 3sahitya karaheNo ratings yet
- Phase Diagram of Fe-Fe3CDocument25 pagesPhase Diagram of Fe-Fe3CIram MustaviNo ratings yet
- Callister7E - pp290 301 (The Iron Carbon System)Document12 pagesCallister7E - pp290 301 (The Iron Carbon System)iglumacNo ratings yet
- FMP 221 Lecture 4Document22 pagesFMP 221 Lecture 4SarojKumarSinghNo ratings yet
- Iron Forms 2Document6 pagesIron Forms 2muralisrikanthNo ratings yet
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- Heat TreatmentDocument179 pagesHeat TreatmentDebye101100% (1)
- TTT Phase DiagramDocument9 pagesTTT Phase Diagramhari krishnaNo ratings yet
- Publication 4 11889 199Document9 pagesPublication 4 11889 199Mulia AridhoNo ratings yet
- Iron-carbon phase diagram guideDocument9 pagesIron-carbon phase diagram guideNagamuthu PandianNo ratings yet
- Lecture 9 - Ferrous AlloysDocument31 pagesLecture 9 - Ferrous Alloysmahmoud foudaNo ratings yet
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- Iron-Carbon DiagramDocument11 pagesIron-Carbon DiagramrampradNo ratings yet
- Lecture 9 - Plain Carbon Steels - 2013Document45 pagesLecture 9 - Plain Carbon Steels - 2013ArunNo ratings yet
- Capili Jefferson 10Document5 pagesCapili Jefferson 10Christian Al EncarnacionNo ratings yet
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatNo ratings yet
- Chapter 8 - Heat TreatmentDocument20 pagesChapter 8 - Heat TreatmentISANo ratings yet
- Metallurgy AssignmentDocument9 pagesMetallurgy Assignmentvishnupriya somaganiNo ratings yet
- Metallurgy AssignmentDocument9 pagesMetallurgy Assignmentvishnupriya somaganiNo ratings yet
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- Theoretical Part: Chapter-1Document10 pagesTheoretical Part: Chapter-1hayder1920No ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (14)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Foundation Science For EngineersDocument7 pagesFoundation Science For EngineersZaid HadiNo ratings yet
- PQR Template (Asme)Document2 pagesPQR Template (Asme)jok1974No ratings yet
- Nanosecond Electromagnetic Pulse Effect On Phase CompositionDocument8 pagesNanosecond Electromagnetic Pulse Effect On Phase CompositionAKNo ratings yet
- Why Is Zero Sequence Impedance of A Shell-Type Transformer Different Than of A Core Type Transformer - QuoraDocument3 pagesWhy Is Zero Sequence Impedance of A Shell-Type Transformer Different Than of A Core Type Transformer - QuoraFilipe Barcelos ResendeNo ratings yet
- Unit ConversionDocument1 pageUnit ConversionHRK65No ratings yet
- Cyclic Triaxial Test Limitations and AdvantagesDocument5 pagesCyclic Triaxial Test Limitations and AdvantagesjobaydaNo ratings yet
- Experimental and Finite Element Analysis On Thermal Conductivity of Fiber Reinforced Building MaterialsDocument10 pagesExperimental and Finite Element Analysis On Thermal Conductivity of Fiber Reinforced Building MaterialsNazish MunirNo ratings yet
- Microscope and Microscopy A New Dimension in DiaDocument1 pageMicroscope and Microscopy A New Dimension in DiaSourab KumarNo ratings yet
- To Estimate Manning's Roughness Coefficient (N) and Chezy's Coefficient (C) For Open Channel Flow.Document6 pagesTo Estimate Manning's Roughness Coefficient (N) and Chezy's Coefficient (C) For Open Channel Flow.Shaheer AhmadNo ratings yet
- Solution Methods For Beam and Frames On Elastic Foundation Using The Finite Element MethodDocument13 pagesSolution Methods For Beam and Frames On Elastic Foundation Using The Finite Element MethodAnonymous 0tT3SeNo ratings yet
- Simple: School of Mechanical and Building SciencesDocument96 pagesSimple: School of Mechanical and Building Sciencessarvesh krishnanNo ratings yet
- Failure of SpringDocument9 pagesFailure of SpringLeandro MarchiNo ratings yet
- Anna Carlmark, Craig Hawker, Anders Hult and Michael Malkoch - New Methodologies in The Construction of Dendritic MaterialsDocument11 pagesAnna Carlmark, Craig Hawker, Anders Hult and Michael Malkoch - New Methodologies in The Construction of Dendritic MaterialsHilltopssNo ratings yet
- TVL ST23 04 Ad0Document9 pagesTVL ST23 04 Ad0coulsonpfilNo ratings yet
- ConductivityDocument36 pagesConductivityThangadurai Senthil Ram PrabhuNo ratings yet
- Properties of Pure SubstanceDocument6 pagesProperties of Pure SubstanceAbid Sufian HusinNo ratings yet
- Cooling of Chips by NanotechnologyDocument16 pagesCooling of Chips by NanotechnologyRupavathi PaulNo ratings yet
- PHY110Document44 pagesPHY110Abhinay YadavNo ratings yet
- Ansys Apdl Short Notes (Er - Suraj Hulke Sir)Document14 pagesAnsys Apdl Short Notes (Er - Suraj Hulke Sir)Er Suraj HulkeNo ratings yet
- Aspen Flare System Analyzer V10 - AspenoneDocument7 pagesAspen Flare System Analyzer V10 - AspenoneMiftah MasrurNo ratings yet
- Unsaturated Soil MechanicsDocument51 pagesUnsaturated Soil MechanicsKrunali Daga100% (1)
- Analysis of Singly Reinforced Concrete Beam PDFDocument3 pagesAnalysis of Singly Reinforced Concrete Beam PDFAnton_Young_1962No ratings yet
- CLT - Design and Use NewDocument58 pagesCLT - Design and Use NewYin LiNo ratings yet
- Ur W28rev2Document25 pagesUr W28rev2Karla JTNo ratings yet
- 11Document9 pages11nyogtNo ratings yet
- ME10 - Heat and Flow 1 - Module DescDocument2 pagesME10 - Heat and Flow 1 - Module DescSridhar TholasingamNo ratings yet
- Segura 2017 Seismic Performance Limitation of Slender Reinforced Concrete Structural WallsDocument268 pagesSegura 2017 Seismic Performance Limitation of Slender Reinforced Concrete Structural WallsDavid VallejoNo ratings yet
- Engg Thermodynamics PDFDocument44 pagesEngg Thermodynamics PDFBrandon FunaNo ratings yet
- Chapter 2Document35 pagesChapter 2tùng thanhNo ratings yet
- 2014-Book-Laske-New Developments in Polymer Composites Research PDFDocument409 pages2014-Book-Laske-New Developments in Polymer Composites Research PDFroxanaNo ratings yet