Professional Documents
Culture Documents
Foods & Human (EE)
Uploaded by
Mike YauOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Foods & Human (EE)
Uploaded by
Mike YauCopyright:
Available Formats
Ch5 Bio / 1
III. Organisms and Environment Essential life processes in animals (Part1A) Foods and human
5.1 Different modes of heterotrophic nutrition Plants can make their own food by photosynthesis. But, most organisms cannot make food themselves. They have to take in organic matter from their surroundings. They depend on other organisms for food supply. The mode of nutrition is called heterotrophic nutrition. Organisms that carry out heterotrophic nutrition = heterotrophs Humans are heterotrophs and are totally dependent on other organisms for their food. Heterotrophic nutrition is divided into 3 main groups: I. Holozoic nutrition Animals like humans & cows carry out this mode of nutrition. They take in organic matter by feeding on other organisms. E.g. (1) Humans obtain food by growing crops, rearing livestock, fishing or hunting. (2) Sheep feeds on grass. II. Saprophytic nutrition Most bacteria & fungi carry out this type of nutrition. They feed on dead organisms / non-living organic matter. They cause decay, e.g. mushrooms. III. Parasitic nutrition Parasites like tapeworms carry out this mode of nutrition. They live on / inside the body of other organisms (hosts) & obtain organic food from them. E.g. Tapeworm lives inside the intestine of humans to obtain food. 5.2 The food requirements of human A The importance of food to us 1. Supply energy Food is oxidized to release energy for supporting metabolism [doing activities] and maintaining body temperature [keeping us warm].
1011 / OUP 1A / 1
Ch5 Bio / 2
2. Support growth and repair Food provides raw materials to support body growth and repair of worn-out tissues of our body. 3. Maintain health Food contains substances that are important for maintaining health [regulate metabolic activities and protect the body against diseases] B The components of food substances There are 7 types of food substances listed below:
Food substances
Primary food substances {essential to life}
Protective food substances {to stay healthy}
Carbohydrates Release energy
Lipids
Proteins
Water
Vitamins
Minerals
Dietary fibre stimulate gut activites
growth+repair transport
maintain health
1 i)
Carbohydrates, lipids and proteins are organic compounds, which are called biomolecules. They contain carbon atoms. Carbohydrates (Gk., Carbo carbon ; hydra water) Carbohydrates are organic substances made up of carbon (C), hydrogen (H) and oxygen (O) atoms. They have a general chemical formula of Cx(H2O)y in which the H:O ratio is 2:1. The basic unit of carbohydrates is simple sugar (saccharide). *All water-soluble carbohydrates are called sugars. According to the no. of sugar molecules, it can be divided into 3 main groups. Monosaccharides (simple sugars) (Gk., mono, one) (CH2O)x Monosaccharides are the simplest forms of carbohydrates. They cannot be hydrolyzed into any simpler carbohydrates. Examples:
1011 / OUP 1A / 2
Ch5 Bio / 3
a. 3-carbon sugars / trioses / (CH2O)3 / C3H6O3 - Important intermediates in respiration and photosynthesis b. 5-carbon sugars / pentoses / (CH2O)5 / C5H10O5 c. 6-carbon sugars / hexoses / (CH2O)6 / C6H12O6 - Glucose, fructose, galactose (i) Taste sweet (ii) Soluble in water (iii) Have reducing power (donate hydrogen/electron to other chemicals and oxidized in the process) reducing sugars - Glucose can be directly broken down to release energy. - It is an immediate energy source for body activities. ii) Disaccharides (double sugars) (C12H22O11) 1. Disaccharides are formed when 2 monosaccharides combine, in the presence of an enzyme. This process is called condensation. During the reaction, 1 water molecule is removed. 2. Disaccharides can be broken down into monosaccharides when water + a different enzyme are present. This reaction is called hydrolysis.
Some common disaccharides are listed below: Types Components Functions / Usage 1. Maltose (malt Glucose + glucose (i) The storage form in some germinating
1011 / OUP 1A / 3
Ch5 Bio / 4
sugar) (ii)
seeds Extracted from barley for brewing and food manufacturing
2. Sucrose (cane sugar) (non-reducing)
Glucose + fructose
(i) (ii)
Table sugar consists of sucrose, which is usually extracted from sugar cane. Abundant in the stems of sugar cane and the roots of sugar beet; source of commercial sugar
3. Lactose (milk sugar)
Glucose + galactose
Present in milk
All are reducing sugars except sucrose. All taste sweet. (sucrose is sweeter than glucose, fructose is 30-40% sweeter than sucrose) All are soluble in water.(sucrose is almost 3 times more soluble than glucose, fructose is the most soluble sugar)
iii) Polysaccharides (multiple sugars) (C6H10O5)n They are long chains of monosaccharides joined together by condensation of many monosaccharides. They are insoluble in water (e.g. starch, glycogen, cellulose) They do not taste sweet (tasteless). They are non-reducing.
1011 / OUP 1A / 4
Ch5 Bio / 5
Types of polysaccharides:(a) Starch It is the storage form of carbohydrates in plants. The large and insoluble nature makes starch an ideal storage form of carbohydrate.
(b) Glycogen It is the storage form of carbohydrate in animals and fungi. It is more soluble than starch and exists in the cytoplasm as tiny granules. It is particularly abundant in liver and muscles of humans. (c) Cellulose It is the main component of plant cell walls. It has a stable structure and strong mechanical strength that makes it an important
1011 / OUP 1A / 5
Ch5 Bio / 6
structural material of the cell wall in plants.
Starch, glycogen and cellulose are all made up of glucose molecules. But, glucose molecules are joined in different ways. Through hydrolysis, polysaccharides are converted into monosaccharides which can be readily used for respiration. Rice, wheat, fruits, noodles, bread and potatoes are rich in starch.
Functions of carbohydrates:They are the main energy source for the body. Each gram of carbohydrate provides about 17.1 kJ of energy. Types Functions** Glucose It is oxidized in cells to release energy for supporting metabolic activities. It affects the water potential of cells and body fluid (e.g. blood). It is important in the regulation of water balance of the body. Glycogen It is the storage form of carbohydrates in liver for regulating the glucose level in blood. It is also stored in muscles as energy reserves to meet the energy need during vigorous muscular activities. Cellulose It is not digested in our body It is an important source of dietary fibre that keeps us healthy, so as to stimulate the peristalsis of gut.
1011 / OUP 1A / 6
Ch5 Bio / 7
Excess of carbohydrates:If too many carbohydrates are consumed, the excess carbohydrates will be converted to glycogen / lipids. Glycogen is stored in the liver and muscles. Lipids are stored under the skin / around the internal organs. 2 Lipids Lipids are also organic substances composed of carbon(C), hydrogen(H) and oxygen(O) atoms. The H : O ratio is much greater than 2:1. E.g. olive oil C57H104O6 ; beef fat C57H110O6 Lipid is formed by the condensation reaction of 3 fatty acids & 1 glycerol molecule. The product is a triglyceride (Gk., tri three ; glycer glycerol) molecule and 3 H2O molecules.
Types of fatty acids:As most naturally occurring lipids contain the same alcohol, namely glycerol, it is the FATTY ACIDS, determining the characteristics of any particular lipid. 1. Carboxyl group () All fatty acids contain a carboxyl group (-COOH) which is partially ionized and can form ionic bonds. The carboxyl group is therefore polar and hydrophilic (water-loving). 2. Hydrocarbon chain The remainder of the molecule is a hydrocarbon chain [CH3(CH2)n] of varying length. This chain is non-polar, hydrophobic / water-repelling. a. The chain may possess one or more double bonds and is said to be unsaturated. These fatty acids have low melting point & are mainly liquid at room temperature. They are OILS and occur mainly in plants.
1011 / OUP 1A / 7
Ch5 Bio / 8
e.g. oleic acid ()C17H33COOH with melting point at 16oC
b. However, the hydrocarbon chain possesses no double bonds, it is said to be saturated. These fatty acids have higher melting points and are solid at room temperature. They are FATS & occur mainly in animals. e.g. palmitic acid () CH3(CH2)14COOH stearic acids () CH3(CH2)16COOH
Types of lipids:1. Fats and oils (i) Most animal lipids are saturated and are in form of FATS that are solids / semi-solids (e.g. pork, beef lard , butter) at room temperature. Taking in too much saturated fatty acids may lead to cardiovascular diseases. (ii) Most plant lipids are unsaturated and are in form of OILS that are liquids (e.g. olive oil , maize oil, corn oil, peanut oil) at room temperature. Plant oil can be hardened to margarine by adding hydrogen to the oil. During the process, the structure of the fatty acid molecules in the oil changes. These fatty acids with changed structure are called TRANS FATS. They are also linked with cardiovascular diseases. Cakes, bread and fried food (e.g. chips) produced by hydrogenated oil
1011 / OUP 1A / 8
(iii)
Ch5 Bio / 9
contain trans fats. 2. Phospholipids Phospholipids are lipids in which one of the fatty acids groups is replaced by phosphate group (PO43-) (from phosphoric acid)
3. Steroids () a. Cholesterol An important component of cell membrane & some hormones Found in the blood Limits the movement of phospholipids in the cell membrane Excessive intake of cholesterol in diet will lead to deposition on arterial wall (inner walls of the blood vessels), thickening the wall (which become less elastic), and leading to a blockage. High risk of heart attacks and stroke will be experienced. b. Steroid is important in the synthesis of steroid hormones such as female sex hormones and vitamin A & D.
Fatty meats, nuts and dairy products are rich in lipids. (e.g. maize, pork, peanuts). Lipids are insoluble in water but dissolve in organic solvents (e.g. ethanol, ether, etc).
Functions / excess of lipids:a. Lipids are also a source of energy. Each g of lipid provides about 38.9 kJ of energy, which is more than twice that of carbohydrates. They provide 25-30% of daily energy requirement of the body. b. Excess lipids will be stored in adipose tissues in the body. These tissues are found under the skin as subcutaneous fat / around the internal organs.
1011 / OUP 1A / 9
Ch5 Bio / 10
(1) They act as food reserve. (2) They act as an insulator to reduce heat loss. (3) Lipids stored around the heart and kidneys act as shock-absorber to protect the internal organs. c. Lipids are a major component of cell membranes. (phospholipids, cholesterol) d. Lipids are involved in transporting and storing fat-soluble vitamins (e.g. vitamins A & D) in the body.
subcutaneous fat
3 Proteins Proteins are organic substances made up of carbon(C), hydrogen(H), oxygen(O) and nitrogen(N). Some also contain sulphur(S), phosphorous(P). Proteins are big molecules which consist of large no. of amino acids. The basic building blocks of proteins are amino acids. 1. Each amino acid molecule contains a carbon atom in the center which carries a carboxyl group (COOH), an amino group (NH2), a side chain (R group) and a hydrogen atom. 2. Each amino acid has its own specific side chain. 3. 20 different amino acids are needed to make up all the different proteins in the body.
When the amino group of an amino acid & the carboxyl group of another are joined by condensation (peptide bond), a dipeptide is formed.
1011 / OUP 1A / 10
Ch5 Bio / 11
Further combination of the dipeptide with other amino acids forms a polypeptide. A protein consists of one / more polypeptide chains. It can be hydrolyzed to amino acids in the presence of H2O and a suitable enzyme. E.g. Insulin is made up of 51 amino acids (C254H317O75N65S6).
coiling and folding
Occurrence of amino acids:Although there are >100 naturally occurring amino acids, only 20 are used in the synthesis of proteins. 1. 8 are essential amino acids because they cannot be made by the human body & have to be taken in diet. (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine) 2. The remaining 12 are non-essential amino acids; they can be made by human body, not essential in human diet. (alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, histidine,
proline, serine, tyrosine)
The 20 different amino acids can be joined together in thousands of different combinations to create different polypeptides or proteins.
1011 / OUP 1A / 11
Ch5 Bio / 12
Types of proteins:1. Fibrous proteins a. They are composed of long & parallel polypeptide chains with regular and repetitive sequence of amino acids. b. These chains are cross-linked to from a fairly stable and insoluble structure so that they form the important building materials of many structures (e.g. nails, hairs). 2. Globular proteins a. They are composed of polypeptides with regular sequence of amino acids. b. These chains are folded into spherical shape. c. They are relatively unstable because their shape is maintained by different types of bonding which are easily denatured by change of environmental conditions. d. Most of them have metabolic functions. (e.g. enzymes, insulin, haemoglobin, antibodies) Properties of proteins:1 Easily denatured by high temperature & extreme pH 2 Slightly soluble in water to form colloidal suspension ()
1011 / OUP 1A / 12
Ch5 Bio / 13
Functions of proteins:a. Proteins are important for the growth and repair of body tissues. Almost all body structures, e.g. muscles & brain tissue, consist of proteins. b. Enzymes, antibodies and some hormones are proteins. [metabolic regulators] 1. Enzymes speed up all the bio-chemical reactions in organisms, e.g. amylase. 2. Antibodies produced by white blood cells defend the body against germs. 3. Hormone regulates physiological processes, e.g. insulin controls the blood glucose level. 4. Haemoglobin in red blood cells increases the oxygen-carrying capacity to transport oxygen to the tissues. c. If carbohydrates and lipids stored in the body are used up [under extreme starvation], proteins may be broken down to provide energy for the body. Each gram of protein provides about 18.2 kJ of energy. Meat, eggs, fish, bean & dairy products are rich in protein.
Excess of proteins: Excess amino acids in the body cannot be stored. They are broken down by the liver. The amino group is removed from the amino acid and changed to urea. This process is called deamination. The urea is passed out in urine. The remaining parts of the amino acids are converted into carbohydrates / lipids. Deficiency of proteins: A lack of protein in diets may lead to a deficiency disease called kwashiorkor. This condition is common in children in developing countries where meat, eggs and milk are limited. Children with kwashiorkor grow poorly. They have a swollen belly because of the accumulation of tissue fluid. 4 Water (H2O) Water has no energy value, but it is essential for life. Water is essential to maintain many life processes in organisms. Water makes up the largest percentage by weight of most organisms. 70% of our body weight is water.
1011 / OUP 1A / 13
Ch5 Bio / 14
Functions of water:1. As a solvent It dissolves various substances and provides the medium for chemical reactions to take place in cells. 2. As a medium for chemical reactions to take place 3. As a transport medium. For example, in form of blood, it transports nutrients, respiratory gases, and metabolic wastes around the body. 4. As a reactant in some metabolic reactions Breaking down certain complex food substances during the digestion of food. i.e. to hydrolyze (digest)food (complex organic substances) into simpler forms. 5. As a cooling agent Water removes heat when it evaporates from the body of an organism. It acts as a cooling agent to help regulate body temperature. E.g. Sweating (perspiration) in humans
Many food and drinks are rich in water.
Excess of water: Humans lose water continuously through sweating, urination, breathing and egestion. Most excess water is passed out from the body as urine. Therefore, we should drink about 6-8 glasses of water every day to replace for the loss.
Vitamins Vitamins are organic food substances (organic compounds) needed in small amounts. They have no energy value, but they help regulate many metabolic reactions. [act as co-enzymes] Some of them can be made by our body while the rest have to be obtained from the diet. * Plants can produce all their vitamins. (i) Vit. B & C are water-soluble, are more easily destroyed by oxygen in air / heat. (ii) Vit. A & D are lipid-soluble, are more heat-resistant. [usually stored in animal fats / plant oils]
1011 / OUP 1A / 14
Ch5 Bio / 15
(1) Vitamin A (fat-soluble/lipid-soluble) (C20H30O)
Daily needs: Functions:
750g Help form a visual pigment in the retina of our eyes (necessary for vision in dim light) Keeps the cornea of eyes, skin, lining of alimentary canal & the breathing system healthy Fish-liver oil Milk Green vegetable (broccoli) Carrot {contain an orange pigment carotene , converted to vitamin A in liver}
Food sources:
Deficiency disease:
a. Poor vision in dim light / night blindness b. Drying up of cornea & skin c. Easy infection of the lining of the lungs & trachea
(2) Vitamin C (ascorbic acid C6H8O6) (water-soluble, easily destroyed by oxygen in air / high temperatures)
Daily needs: Functions:
30g Needed for the growth & repair of connective tissues (help keep body structures in place) Help heal wounds Important to healthy gums & skin
Food sources: Deficiency disease:
Vegetables (e.g. green pepper, broccoli) Fruits (espec. citrus fruits orange, lemon, kiwifruits ; guavas) a. Weak & bleeding gums, resulting in loose teeth b. The body bleeds easily, causing bruises under skin (bruised skin) c. Slow recovery of wounds
SCURVY
Sailors on long voyages without getting fresh fruits / vegetables will have scurvy
(3) Vitamin D (fat-soluble/lipid-soluble) (C28H44O)
Daily needs: Functions:
2.5g Promote the uptake of calcium (Ca2+) & phosphate (PO43) ions from gut Calcium, phosphate ions are essential for keeping bones and teeth strong. Vitamin D is particularly important to actively growing children.
(!!! Vitamin D is not the component of bones)
Food sources: Deficiency disease:
Fish-liver oil Dairy products (milk, butter, egg, fish) The children have weak legs, may bend under the weight of their body)
a. Rickets in children b. Easy bone fractures in adults (with brittle bones) (or even osteoporosis )
1011 / OUP 1A / 15
Ch5 Bio / 16
Absorption
1. Vitamin D can be produced by the skin under sunlight. [ultra-violet light] 2. Calcium-rich food & weight-bearing exercises are useful to slow down calcium loss from bones in adults.
(1 mg = 10 g ; 1g = 10-6 g)
-3
Minerals Minerals are inorganic food substances needed in small amounts. They have no energy value, but are important in regulating many metabolic reactions (e.g. blood clotting) & building body tissues (e.g. bones). We need various minerals, e.g. calcium, iron, iodine, potassium, phosphorus.
Daily needs 500 mg Functions (1) Essential for the formation of bones & teeth Children need a lot of calcium to harden their bones & develop teeth as they grow up. (2) Blood clotting (3) Muscle contraction Deficiency diseases (1) Rickets in children (2) Osteoporosis in adults (3) Bleeding / haemorrhage Food sources Green vegetables (e.g. broccoli) dairy products (e.g. cheese, milk, yoghurt, etc) Functions It is a component of haemoglobin in red blood cells, a pigment in red blood cells that carries oxygen around the body. Deficiency diseases Anaemia with pale face, tired easily and faint because of insufficient haemoglobin to carry oxygen Food sources Beef , meat Liver, kidney Vegetables Yeast, egg
Minerals Calcium (Ca2+)
Iron (Fe2+) (Fe3+)
10 mg
(1 mg = 10-3g)
1011 / OUP 1A / 16
Ch5 Bio / 17
Dietary fibre Dietary fibre (roughage) is an organic food substance, mainly consists of cellulose from plant cell walls. Dietary fibre does not give us energy since we do not have the enzyme to digest it.
Function of dietary fibre: It is essential to our health because it holds a lot of water to enable faeces to remain soft. It also adds bulk to food to stimulate its movement along the alimentary canal (= peristalsis). [facilitate peristalsis of the gut to move food along it] In this way, faeces can be passed out of the body more easily. Plant foods (e.g. vegetables, fruits & cereals) contain dietary fibre.
Deficiency of dietary fibre: Lack of dietary fibre cannot properly stimulate the colon, food moves along the gut very slowly. It causes excessive absorption of water from food. As a result, the faeces become so dry & hard, making egestion very difficult (= constipation). In extreme case, it may lead to intestinal cancer.
5.3 Food tests Based on the chemical properties of food substances, scientists have developed various tests to test for their presence in food. To ensure the +ve result observed is due to the food substance but not other factors, a control using distilled water instead of the sample should be included in the food test. A Detection of food substances by food tests [I] Test for glucose using Clinistix paper (Clinistix paper test) Clinistix paper can be used to detect the presence of glucose in food. It is commonly used to detect glucose in urine. A positive result shows that the person may suffer from diabetes.
Dip the pink test end of Clinistix paper into the sample and observe any colour change. A purple colour indicates the presence of glucose. Negative result no colour change Positive result purple
1011 / OUP 1A / 17
Ch5 Bio / 18
[II] Test for reducing sugars using Benedicts test To test for reducing sugars, e.g. glucose, maltose, fructose.
(water bath)
*Use water bath instead of Bunsen flame Water bath has a better control over the temperature & and can prevent bumping of the mixture.
A brick-red precipitate indicates the presence of reducing sugars. The concentration of reducing sugars can affect the result: Blue (with no glucose present) < green < yellow < orange < red, and then brick red / brown (with high r. sugar present) A colour change would signify the presence of glucose. Summary of reaction:
[III] Test for starch using iodine test Iodine solution detects for the presence of starch by changing its original brown colour to blue-black.
Negative result no colour change
Positive result blue-black colour
1011 / OUP 1A / 18
Ch5 Bio / 19
[IV] Test for lipids using grease spot test 1. Add a drop of cooking oil / egg white solution (control proteins) / distilled water (control) to a filter paper and let it dry in air for 5 mins. 2. Hold up the filter paper to the light & observe any translucent spot. 3. Put the filter paper into an organic solvent (e.g. ether, acetone). Take it out and let it dry. 4. Examine the filter paper against light again.
The disappearance of the spot indicates the presence of lipids. Presence of translucent spot after drying Sample Before immersing into After immersing into organic solvent organic solvent Cooking oil Egg white solution Distilled water --Note:a. The translucent spot caused by lipids is permanent. b. On the contrary, the translucent spot caused by water disappears as water evaporates. c. Lipids but not proteins are soluble in organic solvents. d. Therefore, the translucent spot caused by lipids disappears whereas the one caused by proteins remains on the filter paper. [V] Test for proteins using Albustix paper Albustix paper can be used to detect the presence of proteins in food. It is commonly used to detect proteins in urine. A +ve result shows that the person may suffer from kidney disease.
Dip the yellow test end of Albustix paper into the sample & observe any colour change. A green colour indicates the presence of proteins. Negative result no colour change Positive result green
1011 / OUP 1A / 19
Ch5 Bio / 20
[VI] Test for vitamin C using DCPIP solution The DCPIP (dichlorophenol indophenol) solution in oxidized state is blue. Vitamin C can reduce DCPIP solution and change its colour to become colourless. We should not shake the tube too vigorously; otherwise, oxygen in the air bubbles produced by shaking will lower the reducing power of vitamin C.
Dip the vitamin C solution to the DCPIP until the solution becomes colourless. 1. Boil a test tube of 5 cm3 of vitamin C solution in a water bath for 5 mins. 2. Allow the test tube to cool. 3. Do the test again with the boiled vitamin C solution. - Heat destroys the reducing property of vitamin C, there is no colour change in the solution. - Therefore, we should not boil / overheat fruits or vegetables to prevent the loss of vitamin C. DCPIP is not suitable for detecting the presence of vitamin C in a sample containing glucose. Glucose is reducing & will also decolourize DCPIP, no matter vitamin C is present in the sample or not. B Food tests with different sample in different physical states (1) For solid examples, we have to grind them up with water. (2) Extracts are then obtained by filtering the ground materials. So that we can use the examples to do food tests involving Clinistix paper, Albustix paper, DCPIP solution, etc. (3) Lipids may not come out from some solid examples. (4) We need to grind up the samples and boil them before doing the grease spot test. (5) The food tests can be also used to detect the presence of biomolecules (carbohydrates, lipids and proteins) in living tissues.
1011 / OUP 1A / 20
Ch5 Bio / 21
Summary
Food Reducing sugar Glucose Starch Lipid Test Benedicts Test Clinistix Paper Test Iodine test Grease spot test Procedure Add Benedicts solution (blue) and heat Dip coloured end (pink) to glucose solution Add brown iodine solution to starch Brown blue-black A translucent spot which disappears after dipping in organic solvent. Yellow green Blue colourless Pink purple Positive result Brick-Red precipitate
1. Add on filter paper. 2. Then dip the paper in organic
solvent (ether). Dip the coloured end (yellow) to protein solution Dip the vitamin C into blue DCPIP
Protein Vitamin C
Albustix paper test DCPIP solution
1011 / OUP 1A / 21
Ch5 Bio / 22
5.4 Foodstuffs Different foods contain different food substances in different amounts. Their energy values are also different because of different amounts of carbohydrates, lipids and proteins they contain. 1. The energy values of carbohydrates and proteins are approximately the same. 2. Lipids contain more than twice the energy of carbohydrates / proteins. Food substance Energy value (kJ / g) Carbohydrates 17.1 Lipids 38.9 Proteins 18.2 The table below shows the compositions of some common foods. It tells us the amount of major food substances & energy values in 100g of each food.
1011 / OUP 1A / 22
Ch5 Bio / 23
5.5 A
Balanced diet Defining balanced diet Diet refers to all the food we eat. To maintain health and meet the energy needs of our body, we must have a balanced diet. A BALANCED DIET consists of all the food substances in the right amounts and proportions. To design a balanced diet, dieticians apply 2 concepts: 1. 6 basic food groups 2. Food pyramid The food pyramid is a general guide to having a balanced diet. The amounts of the 6 food groups in a balanced diet are represented by their relative size in the food pyramid.
Principles of healthy eating:a. Eat cereals as the largest portion of food in every meal. b. Eat a lot of vegetables & fruit. c. Eat moderate amount of meat, dairy products & beans. d. Reduce eating food with high salt, fats & sugar content. e. A daily fluid intake of 6-8 glasses (1 glass = 250ml) 2+3 everyday: To keep healthy, it is recommended to have at least 2 servings of fruits + 3 servings of vegetables every day.
1011 / OUP 1A / 23
Ch5 Bio / 24
(a) 1 serving (80g) of fruits is about 2 pieces of small-sized fruits (e.g. plum) / 1 piece of medium-sized fruit (e.g. orange) + 1/2 piece of large-sized fruit (e.g. banana) (b) 1 serving of vegetables is about 1/2 bowl of cooked vegetables.
B (i)
Factors affecting our dietary requirement The balanced diet differs from person to person, depending on a number of factors. Age Children need more energy relative to their body size than adults. They have a larger surface area to volume ratio than adults, therefore a great heat loss. As they grow actively, they need more proteins, calcium & iron for building new tissues. i) Teenagers need more calcium & vitamin D than adults for growth because they are at the stage of active bone growth. ii) Teenagers are at the stage of active growth that need more iron to produce more red blood cells. Sex In general, males need more energy than female. This is because males usually have a larger body size, more muscles (need more energy to contract) & a more active lifestyle than females. Also, there is less subcutaneous fat in males, so they lose heat more rapidly than females. At the same time, female need more iron than males, since they have to produce more red blood cells to replace for the monthly blood loss during menstruation. Level of activity People doing more muscular activities require more energy. A construction worker requires more energy than an office worker. The former needs a diet which is rich in: a. carbohydrates to supply enough energy, and b. proteins for muscle development.
(ii)
(iii)
1011 / OUP 1A / 24
Ch5 Bio / 25
(iv)
Body status (i) Pregnant women have higher daily energy need than a normal woman because extra energy is used to support the development of the foetus. [foetal development] They need more carbohydrates, proteins, minerals & vitamins because their foetuses need energy & raw materials for making new tissues. (ii) Breast-feeding mothers need an extra supply of nutrients in the diet for milk production. Both need more calcium & vitamin D to support the rapid bone growth of the foetus / baby. Both need more iron for the foetus / baby to produce their red blood cells. The daily energy requirements & the recommended intake of food substances for different groups of people are tabulated below.
If the energy uptake is insufficient, the body will oxidize the stored glycogen & lipids to meet the needs. This will cause drop in body mass.
1011 / OUP 1A / 25
Ch5 Bio / 26
Health problems resulting from an improper diet Eating both too much / too little food is unhealthy & can lead to different health problems. 1. Over-nutrition (over-eating) If we eat too much (= the energy intake is greater than the energy needs / consumption / energy consumed), the excess energy will be stored as fats in the body. This will cause a gain in weight. When our body weight exceeds a normal level, we are said to be overweight. The condition of seriously overweight = obesity. Overweight people have a higher risk of developing high blood pressure, heart disease, gall stone, diabetes, etc. Their joints may easily become damaged because of their weight. 2. Under-nutrition (under-eating) If we eat too little (= the energy intake is less than the needs of our body), the glycogen & lipids will be used. In extreme starvation, the proteins in our body will also be consumed. We will have a loss in weight and become thin. Malnutrition / various deficiency diseases will result. Malnutrition is the condition resulting from the lack of one or more of the food substances that are required in the diet to maintain health. (a) Kwashiorkor (protein deficiency disease) Due to the lack of food (especially proteins) Will suffer from stomach distension, muscle wastage, anaemia, retarded growth (b) Eating disorders Some people are improperly on diet without proper medical guidance. So, they are under-weight (too thin) and will seriously affect their health. Their blood glucose level may be below normal & will faint easily. (c) Anorexia nervosa It is another disease of under-eating. It is a kind of mental illness. The patients consider themselves fat even though they are very slim. They fear of putting on weight & refuse to eat (lose appetite). Without proper treatment, they will become dangerously weak. This disease can be fatal.
1011 / OUP 1A / 26
Ch5 Bio / 27
D* STSE Food labels To help consumers make healthy food choices, the HKSAR government has required manufacturers to provide food labels on pre-packaged food since 2007. A food label helps us understand the composition & the energy content of that food. It also provides us with other information like food additives. They are added to prevent food from going bad / give it colour or flavour. Nutrition label must include the information on energy & 7 nutrients specified for labelling (1+7), namely, protein, carbohydrates, total lipids, saturated fatty acids, trans fatty acids, sodium & sugars.
1011 / OUP 1A / 27
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Inflammation Secret Code 1610A Ebook PDFDocument123 pagesThe Inflammation Secret Code 1610A Ebook PDFSADDY100% (3)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Sample Pages From The Low-FODMAP Diet CookbookDocument13 pagesSample Pages From The Low-FODMAP Diet CookbookThe Experiment100% (2)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Liver NafldDocument12 pagesLiver NafldaNo ratings yet
- Fruit Juice CodexDocument19 pagesFruit Juice Codexshahmed999100% (1)
- If T Food ExperimentsDocument63 pagesIf T Food ExperimentsMuhammad Sohail AkramNo ratings yet
- SCIENCE-10 Q4 MOD3 Biomolecules-Carbohydrates-Lipids BookletDocument12 pagesSCIENCE-10 Q4 MOD3 Biomolecules-Carbohydrates-Lipids BookletRetep Aren100% (2)
- Exam1 Sp2014 AnswersDocument6 pagesExam1 Sp2014 AnswersEllen LacbayoNo ratings yet
- The Sugar IndustryDocument22 pagesThe Sugar IndustryAbraham wisdomNo ratings yet
- Chemical Engineering Internship Report at Noon Sugar Mills Ltd. DistilleryDocument59 pagesChemical Engineering Internship Report at Noon Sugar Mills Ltd. DistilleryMuhammad Shahid93% (15)
- Journal of Advanced Research: Yutao Huang, Shuyu Cai, Xiaoli Ruan, Jun Xu, Dongdong CaoDocument13 pagesJournal of Advanced Research: Yutao Huang, Shuyu Cai, Xiaoli Ruan, Jun Xu, Dongdong CaopremicaNo ratings yet
- An Overview of Honey: Its Composition, Nutritional and Functional PropertiesDocument5 pagesAn Overview of Honey: Its Composition, Nutritional and Functional PropertiesSandeep SinghNo ratings yet
- Alcoholic Fermentation Lab ResultsDocument3 pagesAlcoholic Fermentation Lab ResultsMy Roses Are RosèNo ratings yet
- Montesano 2016Document6 pagesMontesano 2016Xanh XanhNo ratings yet
- Chemistry Investigatory Project: Name: Mengulhounuo Keretsu Class: 12 (Science) School: Maple Tree SchoolDocument23 pagesChemistry Investigatory Project: Name: Mengulhounuo Keretsu Class: 12 (Science) School: Maple Tree SchoolmengulhounuoNo ratings yet
- Digestion of CarbohydratesDocument30 pagesDigestion of CarbohydratesRona May EsperanzateNo ratings yet
- CPI - Module 3 - Sugar & StarchDocument17 pagesCPI - Module 3 - Sugar & StarchDinah Jane MartinezNo ratings yet
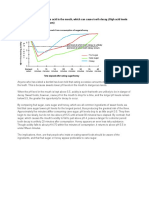
- Eating Sweet Foods Produces Acid in The Mouth, Which Can Cause Tooth Decay. (High Acid Levels Are Measured by Low PH Values)Document19 pagesEating Sweet Foods Produces Acid in The Mouth, Which Can Cause Tooth Decay. (High Acid Levels Are Measured by Low PH Values)Munawir Rahman IslamiNo ratings yet
- foodresearchAS5784880850411521514933715933 Content 1Document8 pagesfoodresearchAS5784880850411521514933715933 Content 1camisha ranny robertNo ratings yet
- Swamy Et. Al. (2010)Document8 pagesSwamy Et. Al. (2010)mpd zulkifliNo ratings yet
- FinalDocument8 pagesFinalapi-246754759No ratings yet
- Ecology of Yeasts in Plant-Bumblebee Mutualism in Central EuropeDocument14 pagesEcology of Yeasts in Plant-Bumblebee Mutualism in Central EuropeSaila Viridiana CazaresNo ratings yet
- C2 PDFDocument19 pagesC2 PDFMiguelAngelGuzmánNo ratings yet
- The Autopol IV Automatic Polarimeter: For Research, Analysis and ControlDocument8 pagesThe Autopol IV Automatic Polarimeter: For Research, Analysis and ControlAgilentechNo ratings yet
- Make stunning chocolate decorationsDocument23 pagesMake stunning chocolate decorationsClary Breganza HaliliNo ratings yet
- Biochem Lab - Handout1Document59 pagesBiochem Lab - Handout1steffiNo ratings yet
- Processing 12 KocherginDocument15 pagesProcessing 12 KocherginJuan MendozaNo ratings yet
- FSHN Exam 2 QuestionsDocument25 pagesFSHN Exam 2 Questionsgradill2No ratings yet
- Carbohydrates, Lipids, Proteins and Nucleic AcidsDocument49 pagesCarbohydrates, Lipids, Proteins and Nucleic Acidssos229072100% (1)
- 14 Natural Ways To Improve Your Insulin SensitiviDocument2 pages14 Natural Ways To Improve Your Insulin SensitiviAshraf ShaikhNo ratings yet
- Clinical Chemistry 2Document65 pagesClinical Chemistry 2Jennifer BaluarteNo ratings yet