Professional Documents
Culture Documents
603 1259 1 PBCVCXV
Uploaded by
Maria AspriOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
603 1259 1 PBCVCXV
Uploaded by
Maria AspriCopyright:
Available Formats
ISSN 0976 3333
Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2012; 3(2):342-347 ORIGINAL RESEARCH ARTICLE
Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
P.K. Senthilkumar*1, D.Reetha1, N.Ramya2 and D.Stella1
2
Department of Microbiology, Annamalai University. Chidambaram 608 002, Tamil Nadu, India Department of Microbiology, Shree Raghavendra Arts and Science College, Keezhamoongiladi, Chidambaram 608 102, Tamil Nadu, India
ABSTRACT The nature of fermented products is different from one region to another. This is depending on the local indigenous microflora, which in turn reflected the climate condition of the area. Mankind has exploited lactic acid bacteria for thousands of years for the production of fermented foods because of their ability to produce desirable changes in taste, flavor and texture as well as inhibit pathogenic and spoilage microorganism. In this present study, the antibacterial potential of lactic acid bacteria and its metabolites was investigated against food borne pathogens. The lactic acid bacteria are isolated by pour plate method obtained from traditional fermented food products such as Curd, Buttermilk, Cheese and Yoghurt. Four lactic acid bacterial isolates viz., Lactobacillus acidophilus, Lactobacillus bulgaricus, Streptococcus thermophilus and Pediococcus acidilactici were identified. The antimicrobial activity of the purified lactic acid bacterial isolates against pathogenic microorganisms viz., Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus were performed by Agar Well Diffusion method and the lactic acid bacterial isolates have the inhibitory activity against bacterial pathogens. Key words: Milk products, Lactic acid bacteria, Food borne pathogens, Antibacterial activity and Well diffusion method. 1. INTRODUCTION Lactic acid bacteria have been used for thousands be easily degraded by living organisms. They are of years in food and alcoholic fermentations. based on renewable raw materials (Protein, oils) Lactic acid bacteria produce various compounds and constitute eco-friendly alternatives to such as organic acids, diacetyl, hydrogen synthetic antimicrobial surfactants. Some of the peroxide, and bacteriocin or bactericidal proteins microbes are best sources of bio-active during lactic fermentations. The bacteriocins from compounds as they synthesize as secondary the lactic acid bacterial isolates generally metabolites for their self defense against other recognized as safe (GRAS) lactic acid bacteria competitive microorganisms [2]. (LAB) have arisen a great deal of attention as a The lactic acid bacteria (LAB) comprise a clade of novel approach to control pathogens in foodGram positive, acid tolerant, non-sporulation, stuffs. Bacteriocins are antimicrobial non-respiring rod or cocci that are associated by proteinaceous compounds that are inhibitory their common metabolic and physiological towards sensitive strains and are produced by both characteristics. These bacteria are usually found in Gram-positive and Gram-negative bacteria. The decomposing plants and lactic products, produce antimicrobial effect of lactic acid bacteria has lactic acid as the major metabolic end product of been appreciated by man for more than 10000 carbohydrate fermentation [3]. This trait has years and has enabled him to extend the shelf life historically linked LAB with food fermentation as [1] of many foods through fermentation processes . acidification inhibits the growth of spoilage Bioactive compounds from plant by-products act agents. The LAB group comprises the genera as good preservatives. Antioxidants poly phenolic Lactobacillus, Streptococcus, Lactococus, fraction from plant by-product are possible Leuconostoc, Pediococcus, Aerococcus, alternatives to synthetic antimicrobial agent can Alloicoccus, Dolosigranulum, Enterococcus, *Corresponding Author: P.K. Senthilkumar, Email: mailtopks@yahoo.com
Received 05 Dec 2011; Revised 15 Mar 2012; Accepted 24 Mar 2012
P.K. Senthilkumar et al. / Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
Globicatella, Lactospaera, Oenococcus, Carnobacterium, Tetragenococcus, Vagoccus and Weissella. Historically, the genera Lactobacillus, Leuconostoc, Pediococcus and Streptococcus form the core of the LAB group [4]. Members of these genera Lactobacillus plays an essential role in the fermentation of food and feed. The most important characteristics of the lactic acid bacteria are their ability to ferment sugars to lactic acid. This may desirable in making products and these organisms have been isolated and screened by using fermented foods such as curd, buttermilk, cheese and yoghurt. Different antimicrobials such as lactic acid, acetic acid, hydrogen peroxide, carbon-di-oxide and bacteriocins produced by these bacteria can inhibit pathogenic and spoilage microorganisms extending the shelf-life and enhancing the safety of food products [5]. Bacteriocins are generally defined as extracellular released peptide or protein that shows a bactericidal activity against more distantly related species. The inhibitory spectrum of some bacteriocins also include food spoilage and for food borne pathogenic microorganisms. The discovery of nisin, the first bacteriocin used on a commercial scale as a food preservative dates back of to the first half of last century but research on bacteriocin of lactic acid bacteria has expanded in the last two decades, searching for novel bacteriocin producing strains from dairy, meat and plant products, as well as traditional fermented products. Among the gram positive bacteria, bacteriocins produced by many lactic acid bacteria used in food fermentation and dairy products, including strains in the genera Lactococcus, Lactobacillus, Pediococcus and Leuconostoc. In USA, only nisin produced by Lactobacillus lactis is permitted as a food preservative [6]. 2. MATERIALS AND METHODS 2.1. Collection of milk product samples The milk product samples viz., Curd, Buttermilk, Cheese and Yoghurt were collected individually in suitable sterile containers from the place of their availability and brought to the laboratory for further study. 2.2. Isolation of lactic acid bacteria The Lactic acid bacteria were isolated from Curd, Buttermilk, Cheese, and Yoghurt by using the MRS Agar medium by Pour plate method. Identification of the bacterial isolates was carried out by the routine bacteriological methods i.e., By the colony morphology, preliminary tests like Gram staining, capsule staining, endospore staining, motility, catalase and oxidase, plating on
2010, IJPBA. All Rights Reserved.
selective medias and performing biochemical tests. 2.3. Maintenance of lactic acid bacterial isolates The Lactic acid bacterial isolates were maintained by sub-culturing routinely in Nutrient Agar slants at every 15 days and stored in refrigerator at 40C. 2.4. Preparation of bacteriocin from lactic acid bacterial isolates After primary screening potential strains were inoculated in MRS broth of 30 ml and kept in a shaker at 150 rpm at 38C. After 24 hours cultures were centrifuged at 3000 rpm for 20 minutes. Supernatant was decanted into sterile test tubes, adjust to pH 6.5-7.0 with a NaOH to remove organic acid effect. H2O2 was neutralized by the addition of catalase from bovine liver at 200/ml. The mixture of the supernatant was filtered with a 0.2m Millipore filter membrane [7]. 2.5. Partial purification of Bacteriocin by Ammonium sulphate precipitation method To the filtrate, 80% Ammonium sulphate precipitation was done (i.e) 14.52 g of Ammonium sulphate was added to dissolve in the culture filtrate and kept in refrigeration overnight for precipitation. This precipitate was dissolved in 2ml of deionized water and dialysed through a 100 molecular weight cut-off dialysis membrane for 48 hours and tested for antimicrobial activity. 2.6. Partial purification of Bacteriocin by solvent extraction method To the filtrate, obtained 70% methanol and 10% Trichloroacetic acid were added in equal proportion and were kept for 48 hours to precipitate at room temperature. This precipitate was dissolved in 2 ml of deionized water and dialysed through a 1000 molecular weight cut-off dialysis membrane for 48 hours and tested for its antimicrobial activity. 2.7. Antimicrobial activity method 2.7.1. Test Organisms Four pathogenic bacteria viz., Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus were used during the present study and were obtained from SGS India laboratories Thoraipakkam, Chennai 96. The cultures were sub-cultured and maintained on Nutrient agar slants and stored at 4C. 2.7.2. Inoculum preparation Bacterial inoculum was prepared by inoculating a loopful of test organisms in 5 ml of Nutrient broth and incubated at 37C for 3-5 hours till a moderate turbidity was developed. The turbidity was matched with 0.5 Mc Farland standards.
343
IJPBA, Mar - Apr, 2012, Vol. 3, Issue, 2
P.K. Senthilkumar et al. / Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
2.7.3. Well diffusion method Sterile Muller Hinton Agar plates were seeded with the indicator organisms by swabbing the entire surface of the culture plates. The holes 6mm in diameter were aseptically punched out of the agar plates, and then 250 1000ml modified MRS broth cultures of the putative organisms were separately introduced into the holes or spotted on to the surfaces of pre poured culture plates and incubated aerobically at 35C for 24 hours. After overnight incubation, inhibition observed by clear zones extending laterally from the border of the putative isolates were noted and recorded in mm diameters. 3. RESULTS AND DISCUSSION LAB was first discovered by Scheele from sour Buttermilk. Pasteur discovered in 1857, that the souring of Buttermilk was caused by the microorganisms. Lactic acid was first produced commercially by M/S Clinton processing company Clinton, Lowa (USA). The universal standard literature accepted for the isolation and characterization of any microorganisms in general and lactic acid bacteria in particular is the Bergeys manual of systemic bacteriology. Lactic acid bacteria found to be associated with various substances via., fermented silages. Isolation and screening of microorganisms from naturally occurring processes have always been the most powerful means for obtaining useful cultures for scientific and commercial purposes. This certainly holds true for Lactic acid bacteria (LAB), which are used throughout the world for manufacture of a wide variety of traditional fermented foods. Marilingappa Hamuna and Kadirvelu [8] Jeevarathnam were isolated and characterized lactic acid bacteria from traditional fermented foods such as appam, batter and Yoghurt. Two Lactobacillus were isolated and used for the extraction of bacteriocin and used as biopreservatives. In the present study, the lactic acid bacteria were isolated from traditional fermented foods such as Curd, Buttermilk, Cheese and Yoghurt and the isolates were Lactobacillus acidophilus, Lactobacillus bulgaricus, Streptococcus thermophilus and Pediococcus acidilactici. In this study, the lactic acid bacteria were characterized from the traditional fermented foods. The lactic acid bacteria were enumerated by pour plate and spread plate method. By the method of Gram staining the morphological characterization were observed under microscope. The conversion of carbohydrates to lactic acid may be well considered as the most important fermentation process employed in food
2010, IJPBA. All Rights Reserved.
technology. However the microbiology and biochemical foundations of lactic fermentation become know only in the course of the last century. As a result, it becomes possible to control this process scientifically and to apply it to modern food technology [9]. The isolated strains were identified on basis of their morphological, physiological and biochemical characteristics, the lactic acid isomer produced, the ability to ferment sugars and 16S rDNA analysis. The genus was Lactobacillus, Streptococcus, Pediococcus, Enterococcus, Leuconostoc and Lactococcus [10]. After morphological identification the identified strains were treated for physiological, carbohydrate and biochemical test. In physiological various temperatures viz., 15, 37 and 45C in broth were determined by visual turbidity after 72 hours incubation and different pH 4.4 and 8.6 were given for their growth. In carbohydrate various sugars Arabinose, Lactose, Maltose, Mannitol, Mannose, Sucrose and Xylose were given to the isolated strains to conform the particular genus and the biochemical test also analyzed for the isolated strains and Oxidase test given the positive result. Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus plantarum, Streptococcus lactis and Streptococcus feacicum had antimicrobial activity. The most sensitivity strains are Staphylococcus aureus was set as a target microorganism. The cultures of Streptococcus facium and Lactobacillus plantarum gave the most intense antimicrobial activity by adding CaCO3 to the medium (to bind accumulated lactic acid) increased the antibiotic activity of the lactic acid bacteria [11]. The bacteriocin of Lactobacillus acidophilus isolated from the milk product which was purified by Ammonium sulphate precipitation was tested for antimicrobial activity. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 23 mm, in Shigella sonnei the inhibition zone was 21 mm, in Bacillus cereus the inhibition zone was 20 mm and in Staphylococcus aureus the inhibition zone was 22 mm. among these results Vibrio cholerae was the best result that was 23 mm and Bacillus cereus shows the lowest result that was 20 mm (Fig 1). The antibacterial activity of Lactobacillus bulgaricus. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholera, the inhibition zone was 22 mm, in Shigella sonnei the inhibition zone was 20 mm. In Bacillus
344
IJPBA, Mar - Apr, 2012, Vol. 3, Issue, 2
P.K. Senthilkumar et al. / Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
cereus, the inhibition zone was 18 mm and in Staphylococcus aureus the inhibition zone was 20 mm. Among these, Vibrio cholerae shows the best result that was 22 mm, and Bacillus cereus showed the lowest result that was 18mm (Fig 2).
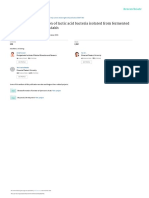
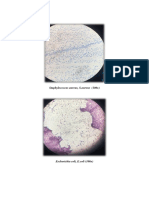
Fig 1: Antimicrobial activity of crude bacteriocin from Lactobacillus acidophilus by ammonium sulphate precipitation method
IJPBA, Mar - Apr, 2012, Vol. 3, Issue, 2
Fig 2: Antimicrobial activity of crude bacteriocin from Lactobacillus bulgaricus by ammonium sulphate precipitation method
The antibacterial activity of bacteriocin obtained and purified by Ammonium sulphate precipitation from Streptococcus thermophilus was investigated. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 20 mm, in Shigella sonnei the inhibition zone was 19 mm, in Bacillus cereus the inhibition zone was 18 mm and in Staphylococcus aureus showed the best result that is 22 mm and Bacillus cereus showed the lowest result that was 18 mm (Fig 3). The antibacterial activity of Pediococcus acidilactici. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae the inhibition zone was 21 mm, in Shigella sonnei, the inhibition zone was 20mm, in Bacillus cereus the inhibition zone was 18 mm and in Staphylococcus aureus the inhibition zone was 20 mm. Among these results, Vibrio cholerae showed the best
2010, IJPBA. All Rights Reserved.
result that was 21 mm, and Bacillus cereus showed the lowest result that was 18mm (Fig 4). Lactobacillus was isolated from MRS and LAPTg (Lactose Propyl Thiogalactosidase) broth and their antimicrobial activity was tested against the test strains using the disc diffusion method. All isolated, except Escherichia coli 0157:H7, showed additional 3 to 4 mm of inhibition zone. This was < 3 mm for Escherichia coli 0157: H7. Lactobacillus isolates were the inhibitor to the test strains followed by Pediococcus, Streptococcus and Leconostoc. Escherichia coli 0157:H7 was the least sensitive in all cased [12]. The antimicrobial activity was determined for the identified strains such as Lactobacillus acidophilus, Lactobacillus bulgaricus, Streptococcus thermophilus and Pediococcus acidilactici. Bacteriocins activity was precipitated by ammonium sulfate extraction method showed maximum activity compared to solvent extraction method. The (Fig 5) showed the results of antibacterial activity of Lactobacillus acidophilus which was obtained by solvent extraction method. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 21 mm, in Shigella sonnei, the inhibition zone was 19 mm, in Bacillus cereus the inhibition zone was 18 mm and in Staphylococcus aureus the inhibition zone was 19 mm. Among these results Vibrio cholerae showed the best result that is 21 mm, and Bacillus cereus showed the lowest result that is 18mm. The (Fig 6) showed the results of antimicrobial activity Lactobacillus bulgaricus which was obtained by solvent extraction method. The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 20 mm, in Shigella sonnei the inhibition zone was 19 mm, in Bacillus cereus the inhibition zone was 16 mm and in Staphylococcus aureus the inhibition zone was 19 mm. Among these results, Vibrio cholerae shows the best result that is 20 mm, and Bacillus cereus shows the lowest result that was 16mm. The antibacterial activity of Streptococcus thermophilus which was obtained by solvent extraction method was showed in (Fig 7). The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 19 mm, in Shigella sonnei the inhibition zone is 18 mm, in Bacillus cereus the inhibition zone was 15 mm and in Staphylococcus aureus the inhibition zone was 18 mm. Among these results, Vibrio cholerae shows the best result that was 20 mm, and
345
P.K. Senthilkumar et al. / Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
Bacillus cereus showed the lowest result that was 15mm. The results of antibacterial activity of Pediococcus acidilactici which was obtained by solvent extraction method was showed in (Fig 8). The test strains are Vibrio cholerae, Shigella sonnei, Bacillus cereus and Staphylococcus aureus. In Vibrio cholerae, the inhibition zone was 19 mm, in Shigella sonnei the inhibition zone was 18 mm, in Bacillus cereus the inhibition zone was 14 mm and in Staphylococcus aureus the inhibition zone was 18 mm. Among these results Vibrio cholerae shows the best result that was 19 mm, and Bacillus cereus shows the lowest result that was 14mm. From this study, it is concluded that the bacteriocin produced by Lactic acid bacteria can be applied both in, food industry and medical sector. They can be used in cheese, fermented sausage, beer, etc., yielding food quality and food safety advantages. Bacteriocins of Lactic acid bacteria can play a role in human gastrointestinal track, hence contributing to human health. IJPBA, Mar - Apr, 2012, Vol. 3, Issue, 2
Fig 3: Antimicrobial activity of crude bacteriocin from Streptococcus thermophilus by ammonium sulphate precipitation method
Fig 5: Antimicrobial activity of crude bacteriocin from Lactobacillus acidophilus by solvent extraction method
Fig 6: Antimicrobial activity of crude bacteriocin from Lactobacillus bulgaricus by solvent extraction method
Fig 7: Antimicrobial activity of crude bacteriocin from Streptococcus thermophilus by solvent extraction method
Fig 4: Antimicrobial activity of crude bacteriocin from Pediococcus acidilactici by ammonium sulphate precipitation method
Fig 8: Antimicrobial activity of crude bacteriocin from Pediococcus acidilactici by solvent extraction method
2010, IJPBA. All Rights Reserved.
346
P.K. Senthilkumar et al. / Antibacterial Potential of Lactic Acid Bacteria and Its Metabolites against Food Borne Pathogens
REFERENCES 1. Padmanabha Reddy, V, M.D. Christopher and I. Sankara Reddy. 2006. Antimicrobial activity of Lactobacillus acidophilus. J. Veterinary and Animal Sciences, 2(4): 142-144. 2. Michel Bakar Diop, Robin DuboisDauphin, Emmanuel Tine, Abib Ngom, Jacqueline Destain, Philippe Thonart, 2007. Bacteriocin producers from traditional food products. Biotechnol. Agron. Soc. Environ., 11:275-281. 3. Nowroozi J, M. Mirzaii and M. Norouzi. 2004. Study of Lactobacillus as probiotic bacteria. Iranian J. Public Health, 33: 1-7. 4. Ko, S.H and C. Ahn. 2000. Bacteriocin production by Lactococcus lactis KCA 2386 isolated from white kimchi. Food Sci. Biotechnol., 9: 263-269. 5. Gobben, G.J, I. Cain-Joe, V.A. Kitzen, I.C. Boels, F. Boer, J.A.M. De Bont. 1998. Enhancement of exopolysaccharide production by Lactobacillus delbrueckii sub sp. bulgaricus NCFB 2772 with a simplified defined medium. Appl. Environ. Microbial., 64(4): 1333-1337. 6. Eva Rodriguez, Juan L. Arques, Manuel Nunez, Pilar Gaya and Margarita Medina. 2005. Combined effect of High-Pressure treatments and bacteriocin producing LAB on inactivation of E. coli in raw milk cheese. Applied Environmental Microbiology, 3399-3404.
IJPBA, Mar - Apr, 2012, Vol. 3, Issue, 2
7. Adetunji, V.O and G.O. Adegoke, 2007. Bacteriocin and cellulose production by Lactic acid bacteria isolated from West African soft Cheese. African J. Biotechnol., 6: 2616-2619. 8. Marilingappa Hamuna and Kadirvelu Jeevarathnam. 2004. Mode of action of Lactococcin B, a thiol activated bacteriocin from Lactococcus lactis. Appl. Environ. Microbial., 59(4) 1041-1048. 9. Kandler O. 1983. Carbohydrates metabolism in Lactic acid bacteria. Antonic Van Leewenhook, 49: 209-224. 10. Harun Ur Rashid, Kaname Togo and Minoru Ueda, Taku Miyamoto. 2007. Identification and characterization of dominant Lactic acid bacteria isolated from traditional fermented milk Dahi in Bangaladesh. J. Microbial biotechnol., 23 125-133. 11. Frederic Lerory and Luc De Vuyst. 2004. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends food sci. Technol., 15: 67-68. 12. Girum Tadesse, Eden Ephraim and Mogessie Ashenafr. 2003. Assessment of the antimicrobial activity of lactic acid bacteria isolated from Borde and Shamitta traditional Ethiopian fermented beverages, on some food-borne pathogens and effect of growth medium on the inhibitory activity. International Journal of Food Safety, 5: 13-20.
2010, IJPBA. All Rights Reserved.
347
You might also like
- Starter Cultures in Food ProductionFrom EverandStarter Cultures in Food ProductionBarbara SperanzaNo ratings yet
- Bacteriocins From Lactic Acid Bacteria Inhibit Food Borne Pathogens.Document7 pagesBacteriocins From Lactic Acid Bacteria Inhibit Food Borne Pathogens.International Organization of Scientific Research (IOSR)No ratings yet
- In-Vitro Screening of Antibacterial Activity of Lactic Acid Bacteria Against Common Enteric PathogensDocument6 pagesIn-Vitro Screening of Antibacterial Activity of Lactic Acid Bacteria Against Common Enteric PathogensInternational Medical PublisherNo ratings yet
- Research Article: in Vitro Evaluation of Probiotic Properties of Lactic Acid BacteriaDocument12 pagesResearch Article: in Vitro Evaluation of Probiotic Properties of Lactic Acid BacteriaNatalija Atanasova-PancevskaNo ratings yet
- Bacteriocin Producing Lactic Acid Bacteria from South Indian Appam BatterDocument8 pagesBacteriocin Producing Lactic Acid Bacteria from South Indian Appam BatterBenita ChristinaNo ratings yet
- Extraction of Bacteriocin and Study of Its Antigonastic AssayDocument6 pagesExtraction of Bacteriocin and Study of Its Antigonastic AssayCrois NadeelNo ratings yet
- Characterisation of Bacteriocins Produced by LactoDocument10 pagesCharacterisation of Bacteriocins Produced by Lactokhurry testNo ratings yet
- Jawan 2019 J. Phys. Conf. Ser. 1358 012020 PDFDocument13 pagesJawan 2019 J. Phys. Conf. Ser. 1358 012020 PDF温洁胜No ratings yet
- Journal Kimchi 1Document11 pagesJournal Kimchi 1DwiNo ratings yet
- Laboratory Exercise 2 - Lactic Acid BacteriaDocument2 pagesLaboratory Exercise 2 - Lactic Acid BacteriaMA. NECOLE JEREMIAH GONZALESNo ratings yet
- Lactobacillus acidophilus NIT Probiotic CharacteristicsDocument5 pagesLactobacillus acidophilus NIT Probiotic CharacteristicsAfdhalRuslanNo ratings yet
- Abr 2010 1 4 218 228Document11 pagesAbr 2010 1 4 218 228Ana-Maria SiminescuNo ratings yet
- Isolation and Molecular Study of Potentially Probiotic Lactobacilli in Traditional White Cheese of Tabriz in IranDocument4 pagesIsolation and Molecular Study of Potentially Probiotic Lactobacilli in Traditional White Cheese of Tabriz in IranBelfas Giovano RamadhanNo ratings yet
- Pruebas Bioquimicas de LactobacillusDocument8 pagesPruebas Bioquimicas de LactobacillusSergio Contreras ReinosaNo ratings yet
- Lactic Acid Bacteria Isolation From Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties EvaluationDocument22 pagesLactic Acid Bacteria Isolation From Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties EvaluationPalupi Cahyaning RatriNo ratings yet
- Food Fermentation With The Help of Microbiology. 1: (Noor Ul Huda) (Monday/16/April/2018)Document7 pagesFood Fermentation With The Help of Microbiology. 1: (Noor Ul Huda) (Monday/16/April/2018)noor hudaNo ratings yet
- Screening For Potential Probiotic From Spontaneously Fermented Non-Dairy Foods Based On in Vitro Probiotic and Safety PropertiesDocument11 pagesScreening For Potential Probiotic From Spontaneously Fermented Non-Dairy Foods Based On in Vitro Probiotic and Safety PropertiesRaul BarradasNo ratings yet
- 09 2806 Research0205 63 75 PDFDocument13 pages09 2806 Research0205 63 75 PDFviesNo ratings yet
- Isolation of Lactic Acid Bacteria With High Biological Activity From Local Fermented Dairy ProductsDocument8 pagesIsolation of Lactic Acid Bacteria With High Biological Activity From Local Fermented Dairy ProductsEnkhbaatar BatmagnaiNo ratings yet
- 1 s2.0 S0308814618308148 MainDocument9 pages1 s2.0 S0308814618308148 MainAusteridad LopezNo ratings yet
- BBRC Vol 14 No 04 2021-20Document7 pagesBBRC Vol 14 No 04 2021-20Dr Sharique AliNo ratings yet
- Kefir Bakteriyosin BacteriocinDocument6 pagesKefir Bakteriyosin Bacteriocinsezer01No ratings yet
- Lactic Acid Production Using Lactic Acid Bacteria Under Optimized ConditionsDocument6 pagesLactic Acid Production Using Lactic Acid Bacteria Under Optimized ConditionsHanan HusseiniNo ratings yet
- Lactic Acid Bacteria: Food Safety and Human Health ApplicationsDocument31 pagesLactic Acid Bacteria: Food Safety and Human Health ApplicationsANGEL SAUL SERRANO ANGULONo ratings yet
- Isolation and Partial Purification of Bacteriocins From Milk & Milk ProductsDocument26 pagesIsolation and Partial Purification of Bacteriocins From Milk & Milk ProductsGaurav Dogra100% (1)
- Isolation and Production of Bacteriocin by Marine Lactobacillus Fermentum SBS001Document8 pagesIsolation and Production of Bacteriocin by Marine Lactobacillus Fermentum SBS001FerryVernandoNo ratings yet
- Identification of Lactobacillus acidophilus using Biolog systemDocument5 pagesIdentification of Lactobacillus acidophilus using Biolog systemarina31No ratings yet
- Cloning and Expression of Bacteriocins of Pediococcus SPP.: A ReviewDocument18 pagesCloning and Expression of Bacteriocins of Pediococcus SPP.: A ReviewInternational Medical PublisherNo ratings yet
- Ijfs2022 2076021Document15 pagesIjfs2022 2076021hibamo bereketNo ratings yet
- Phylogenetic analysis of acid tolerant gut bacteria from two prawn speciesDocument16 pagesPhylogenetic analysis of acid tolerant gut bacteria from two prawn speciesVedjia MedhytaNo ratings yet
- Holz-LAB-antifungalDocument17 pagesHolz-LAB-antifungalIstván NagyNo ratings yet
- Probiotic Properties of Lactobacillus Helveticus and Lactobacillus Plantarum Isolated From Traditional Pakistani YoghurtDocument17 pagesProbiotic Properties of Lactobacillus Helveticus and Lactobacillus Plantarum Isolated From Traditional Pakistani Yoghurtkhurry testNo ratings yet
- BacteriocinDocument16 pagesBacteriocintransformers7No ratings yet
- 1 Pilihan Hari JugaDocument9 pages1 Pilihan Hari JuganadyasuwayviaNo ratings yet
- Jurnal 2Document7 pagesJurnal 2SasaNo ratings yet
- Characterization of Bacillus megaterium and Bacillus mycoides as probiotic bacteriaDocument9 pagesCharacterization of Bacillus megaterium and Bacillus mycoides as probiotic bacteriaAusteridad LopezNo ratings yet
- Isolation and MolecularDocument6 pagesIsolation and MolecularRasendriya Edra DaniswaraNo ratings yet
- 6 - Antioxidant Activity of Bifidobacterium Animalis MSMC83 and Its ApplicationDocument9 pages6 - Antioxidant Activity of Bifidobacterium Animalis MSMC83 and Its ApplicationJazmin BelloNo ratings yet
- Djide - 2020 - IOP - Conf. - Ser. - Earth - Environ. - Sci. - 575 - 012035Document6 pagesDjide - 2020 - IOP - Conf. - Ser. - Earth - Environ. - Sci. - 575 - 012035yusufitriNo ratings yet
- BacteriocinsDocument3 pagesBacteriocinsKhushi VermaNo ratings yet
- C0 Formulir PemohonanDocument5 pagesC0 Formulir PemohonanDiah AyuningrumNo ratings yet
- Kefir de AguaDocument24 pagesKefir de AguaanaymgalvizNo ratings yet
- Isolation and Characterization of Lactobacillus From Curd and Its Application in Food IndustryDocument9 pagesIsolation and Characterization of Lactobacillus From Curd and Its Application in Food IndustryIJRASETPublicationsNo ratings yet
- 070 - Rha'idah Madihah - Jurnal Internasional - TekferDocument6 pages070 - Rha'idah Madihah - Jurnal Internasional - TekferNajma SalisaNo ratings yet
- Screening and Production of Protease Enzyme From Marine Microorganism and Its Industrial ApplicationDocument9 pagesScreening and Production of Protease Enzyme From Marine Microorganism and Its Industrial ApplicationIOSRjournalNo ratings yet
- In-Vitro Anti-Food Poisoning Bacteria and Cytotoxic Activities of Bioactive Compounds Produced by Streptomyces Sp. A10 Isolated From Fermented FoodDocument9 pagesIn-Vitro Anti-Food Poisoning Bacteria and Cytotoxic Activities of Bioactive Compounds Produced by Streptomyces Sp. A10 Isolated From Fermented FoodSabrina JonesNo ratings yet
- Fermeted Juice ProbioticDocument6 pagesFermeted Juice ProbioticOlfa Ben MoussaNo ratings yet
- LAB ImporranceDocument9 pagesLAB ImporranceNaveed AhmadNo ratings yet
- Antibacterial Metabolites of Lactic Acid Bacteria: Their Diversity and PropertiesDocument15 pagesAntibacterial Metabolites of Lactic Acid Bacteria: Their Diversity and PropertiesHanaNo ratings yet
- RPB14150077015Document7 pagesRPB14150077015Ijupbs IjupbsNo ratings yet
- Reuteri 2-20B and Pediococcus Acidilactici 0-11A Isolated From FuraDocument9 pagesReuteri 2-20B and Pediococcus Acidilactici 0-11A Isolated From FuramostecocNo ratings yet
- MoleculesDocument13 pagesMoleculesMonyet...No ratings yet
- Antimicrobial potato juice boosts calf healthDocument10 pagesAntimicrobial potato juice boosts calf healthDavien UtoyoNo ratings yet
- BacteriosinDocument5 pagesBacteriosinSayu Putu Yuni ParyatiNo ratings yet
- ID Senyawa Antibakteri Yang Diproduksi OlehDocument8 pagesID Senyawa Antibakteri Yang Diproduksi OlehNurawaliah RezkyNo ratings yet
- Rastogi2019 Article InVitroEvaluationOfProbioticPoDocument12 pagesRastogi2019 Article InVitroEvaluationOfProbioticPoMaria AspriNo ratings yet
- Analysis of Antimicrobial Activity of Lactobacillus Paracasei Ssp. Paracasei-1 Isolated From Regional YogurtDocument11 pagesAnalysis of Antimicrobial Activity of Lactobacillus Paracasei Ssp. Paracasei-1 Isolated From Regional YogurtTarif_87No ratings yet
- JMB019 02 11Document9 pagesJMB019 02 11Nure Junnatul HemaniNo ratings yet
- LWT PPRDocument9 pagesLWT PPRAayushi BalyanNo ratings yet
- Microorganisms 08 00354 v2Document21 pagesMicroorganisms 08 00354 v2ishita modasiyaNo ratings yet
- Cellular Respiration ProtocolDocument7 pagesCellular Respiration ProtocolMuhammad EvanNo ratings yet
- FM CansGetYouCooking Pea Infographic 2018Document1 pageFM CansGetYouCooking Pea Infographic 2018Maria AspriNo ratings yet
- HAYATI Journal of Biosciences: Amal Bakr ShoriDocument5 pagesHAYATI Journal of Biosciences: Amal Bakr ShoriMaria AspriNo ratings yet
- 751149v1 Full PDFDocument30 pages751149v1 Full PDFMaria AspriNo ratings yet
- Why manuscripts are rejected from an education journalDocument9 pagesWhy manuscripts are rejected from an education journalVijay Sri ThiruNo ratings yet
- FST MCQS 1 With Answers KeyDocument61 pagesFST MCQS 1 With Answers KeyAleem Muhammad87% (15)
- Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A ReviewDocument15 pagesStructural Stability and Viability of Microencapsulated Probiotic Bacteria: A ReviewMaria AspriNo ratings yet
- Kalfes@escpeurope - Eu A.shantz@ieseg - FR R.Alahakone@kingston - Ac.ukDocument2 pagesKalfes@escpeurope - Eu A.shantz@ieseg - FR R.Alahakone@kingston - Ac.ukMaria AspriNo ratings yet
- Rated Review ExamplesDocument41 pagesRated Review ExamplesMaria AspriNo ratings yet
- 10 1016@j Ifset 2014 09 010 PDFDocument35 pages10 1016@j Ifset 2014 09 010 PDFMaria AspriNo ratings yet
- Mcinnis Food Micro 46 121 131Document11 pagesMcinnis Food Micro 46 121 131Maria AspriNo ratings yet
- Rastogi2019 Article InVitroEvaluationOfProbioticPoDocument12 pagesRastogi2019 Article InVitroEvaluationOfProbioticPoMaria AspriNo ratings yet
- FST MCQS 1 With Answers KeyDocument61 pagesFST MCQS 1 With Answers KeyAleem Muhammad87% (15)
- Investigation of Bacterial and Fungal Diversity in Tarag Using High-Throughput SequencingDocument12 pagesInvestigation of Bacterial and Fungal Diversity in Tarag Using High-Throughput SequencingMaria AspriNo ratings yet
- Nayak2020 Article Physico-chemicalCompositionMin PDFDocument8 pagesNayak2020 Article Physico-chemicalCompositionMin PDFMaria AspriNo ratings yet
- Nayak2020 Article Physico-chemicalCompositionMin PDFDocument8 pagesNayak2020 Article Physico-chemicalCompositionMin PDFMaria AspriNo ratings yet
- Lysozyme A New Allergen in Donkey S Milk PDFDocument7 pagesLysozyme A New Allergen in Donkey S Milk PDFMaria AspriNo ratings yet
- Sarti2019 Article DonkeySMilkInTheManagementOfCh PDFDocument9 pagesSarti2019 Article DonkeySMilkInTheManagementOfCh PDFMaria AspriNo ratings yet
- Donkey Milk and Dairy Donkey Farming in Mediterranean Countries Current Situation, Challenges and ProspectsDocument27 pagesDonkey Milk and Dairy Donkey Farming in Mediterranean Countries Current Situation, Challenges and ProspectsMaria AspriNo ratings yet
- 751149v1 Full PDFDocument30 pages751149v1 Full PDFMaria AspriNo ratings yet
- 751149v1 Full PDFDocument30 pages751149v1 Full PDFMaria AspriNo ratings yet
- Whey Proteins and Their Antimicrobial Properties in Donkey Milk: A Brief ReviewDocument15 pagesWhey Proteins and Their Antimicrobial Properties in Donkey Milk: A Brief ReviewMaria AspriNo ratings yet
- Laboratory Manual in General Microbiology For Undergraduate Students., ShortDocument49 pagesLaboratory Manual in General Microbiology For Undergraduate Students., ShortMaria AspriNo ratings yet
- 37-Article Text-483-1-10-20191024 PDFDocument3 pages37-Article Text-483-1-10-20191024 PDFMaria AspriNo ratings yet
- Whey Proteins and Their Antimicrobial Properties in Donkey Milk: A Brief ReviewDocument15 pagesWhey Proteins and Their Antimicrobial Properties in Donkey Milk: A Brief ReviewMaria AspriNo ratings yet
- Beverages 05 00014 v2Document14 pagesBeverages 05 00014 v2Maria AspriNo ratings yet
- Lysozyme A New Allergen in Donkey S Milk PDFDocument7 pagesLysozyme A New Allergen in Donkey S Milk PDFMaria AspriNo ratings yet
- 37-Article Text-483-1-10-20191024 PDFDocument3 pages37-Article Text-483-1-10-20191024 PDFMaria AspriNo ratings yet
- Microbial GrowthDocument41 pagesMicrobial GrowthMaria AspriNo ratings yet
- 2015food Tech WDocument21 pages2015food Tech WNirmal SharmaNo ratings yet
- What Microorganisms Are Deactivated by UltravioletDocument2 pagesWhat Microorganisms Are Deactivated by UltravioletBurak UçkaçNo ratings yet
- Lab 3Document6 pagesLab 3Diyana Nabila Abdul WahidNo ratings yet
- ENTEROBACTERIACEAEDocument23 pagesENTEROBACTERIACEAEapi-19916399No ratings yet
- Microbiology 1Document35 pagesMicrobiology 1pikachuNo ratings yet
- Biological Classification 2Document2 pagesBiological Classification 2Dr. D. kumar Harshit -apexNo ratings yet
- Certificate of Analysis: Test Items Specifications ResultsDocument1 pageCertificate of Analysis: Test Items Specifications ResultsCretulescu CameliaNo ratings yet
- EnterobakterDocument36 pagesEnterobakterUttari DalemNo ratings yet
- Annexure 4 Colony Morphology, Shape & Cell Arrangment of MicroorganismDocument6 pagesAnnexure 4 Colony Morphology, Shape & Cell Arrangment of Microorganismdinesh singhNo ratings yet
- (Methods in Microbiology 17) P.M. Bennett and J. Grinsted (Eds.) - Plasmid Technology-Academic Press (1984) PDFDocument320 pages(Methods in Microbiology 17) P.M. Bennett and J. Grinsted (Eds.) - Plasmid Technology-Academic Press (1984) PDFStella RodicaNo ratings yet
- Assignment PDFDocument6 pagesAssignment PDFaubrey yangzonNo ratings yet
- Micro MCQ W AnswersDocument41 pagesMicro MCQ W AnswersDiana Ioana100% (1)
- Microbiology PosterDocument1 pageMicrobiology PosterAgnieszkaNo ratings yet
- Gram Positive Bacteria of Medical ImportanceDocument12 pagesGram Positive Bacteria of Medical ImportanceGeorge C. KasondaNo ratings yet
- Diversity of BacteriaDocument3 pagesDiversity of BacteriaLisa AllisyaNo ratings yet
- Micro Unknown Lab ReportDocument12 pagesMicro Unknown Lab ReportTika Ram100% (5)
- Media Farmasi P.issn 0216-2083 E.issn 2622-0962 Vol. XV No. 1, April 2019Document6 pagesMedia Farmasi P.issn 0216-2083 E.issn 2622-0962 Vol. XV No. 1, April 2019Ivanda PrilsciliaNo ratings yet
- 1 Bacte Mtap 1Document12 pages1 Bacte Mtap 1DENISE MARA�ANo ratings yet
- Culture MediaDocument6 pagesCulture MediaMeiYeng,rmtNo ratings yet
- Neisseria Gonorrhea SlideDocument8 pagesNeisseria Gonorrhea SlideNURIZATI AYIENo ratings yet
- Principle Mode of Action SpecimenDocument2 pagesPrinciple Mode of Action SpecimensrrabelloNo ratings yet
- The Antibacterial Activity of Mahkota Dewa Leaf Infusion Against Staphylococcus Aureus and Eschericia ColiDocument6 pagesThe Antibacterial Activity of Mahkota Dewa Leaf Infusion Against Staphylococcus Aureus and Eschericia ColiSintya suci dwiyanaNo ratings yet
- Haemophilus, HACEK, Anaerobes HANDOUTSDocument57 pagesHaemophilus, HACEK, Anaerobes HANDOUTSEkhizz VicenteNo ratings yet
- Microbiology Lab Test 2 Review: Acid Fast Stain, KOH, India Ink, LPCB, Gram StainDocument5 pagesMicrobiology Lab Test 2 Review: Acid Fast Stain, KOH, India Ink, LPCB, Gram StainRoyNo ratings yet
- )Document6 pages)nuraninarunNo ratings yet
- Result:: Staphylococcus Aureus, S.aureus (100x)Document3 pagesResult:: Staphylococcus Aureus, S.aureus (100x)Ayuni Nadrah Bt KamarujamanNo ratings yet
- Journal Reading: Endoscopically-Derived Bacterial Cultures in Chronic Rhinosinusitis: A Systematic ReviewDocument21 pagesJournal Reading: Endoscopically-Derived Bacterial Cultures in Chronic Rhinosinusitis: A Systematic ReviewCahya AlfalizaNo ratings yet
- 1 IntroDocument5 pages1 IntroJeanjayannseptoemanNo ratings yet
- Microbiology Practical Exam NotesDocument14 pagesMicrobiology Practical Exam NotesTovin Nguyen100% (1)
- Microbiology 1st Edition Wessner Solutions ManualDocument7 pagesMicrobiology 1st Edition Wessner Solutions Manuala424161136No ratings yet
- Introduction To BacteriologyDocument1 pageIntroduction To BacteriologySeon u 'No ratings yet
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessFrom Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessRating: 4 out of 5 stars4/5 (33)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceFrom EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceRating: 4.5 out of 5 stars4.5/5 (515)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindFrom EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindRating: 4.5 out of 5 stars4.5/5 (93)
- Crypt: Life, Death and Disease in the Middle Ages and BeyondFrom EverandCrypt: Life, Death and Disease in the Middle Ages and BeyondRating: 4 out of 5 stars4/5 (3)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- This Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyFrom EverandThis Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyRating: 3.5 out of 5 stars3.5/5 (31)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionFrom EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionRating: 4 out of 5 stars4/5 (811)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesFrom EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesRating: 4.5 out of 5 stars4.5/5 (397)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorFrom EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNo ratings yet
- Wayfinding: The Science and Mystery of How Humans Navigate the WorldFrom EverandWayfinding: The Science and Mystery of How Humans Navigate the WorldRating: 4.5 out of 5 stars4.5/5 (18)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedFrom EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedRating: 4 out of 5 stars4/5 (11)
- The Lives of Bees: The Untold Story of the Honey Bee in the WildFrom EverandThe Lives of Bees: The Untold Story of the Honey Bee in the WildRating: 4.5 out of 5 stars4.5/5 (44)
- The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and IntestineFrom EverandThe Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and IntestineRating: 4 out of 5 stars4/5 (17)
- Eels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishFrom EverandEels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishRating: 4 out of 5 stars4/5 (30)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsFrom EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsRating: 4.5 out of 5 stars4.5/5 (4)
- Human Errors: A Panorama of Our Glitches, from Pointless Bones to Broken GenesFrom EverandHuman Errors: A Panorama of Our Glitches, from Pointless Bones to Broken GenesRating: 3.5 out of 5 stars3.5/5 (56)
- The Mind & The Brain: Neuroplasticity and the Power of Mental ForceFrom EverandThe Mind & The Brain: Neuroplasticity and the Power of Mental ForceNo ratings yet
- Gathering Moss: A Natural and Cultural History of MossesFrom EverandGathering Moss: A Natural and Cultural History of MossesRating: 4.5 out of 5 stars4.5/5 (347)
- The Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsFrom EverandThe Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsNo ratings yet
- Younger for Life: Feel Great and Look Your Best with the New Science of AutojuvenationFrom EverandYounger for Life: Feel Great and Look Your Best with the New Science of AutojuvenationRating: 4 out of 5 stars4/5 (1)
- Darwin's Dangerous Idea: Evolution and the Meaning of LifeFrom EverandDarwin's Dangerous Idea: Evolution and the Meaning of LifeRating: 4 out of 5 stars4/5 (523)
- Superlative: The Biology of ExtremesFrom EverandSuperlative: The Biology of ExtremesRating: 4.5 out of 5 stars4.5/5 (51)
- Why We Sleep: Unlocking the Power of Sleep and DreamsFrom EverandWhy We Sleep: Unlocking the Power of Sleep and DreamsRating: 4.5 out of 5 stars4.5/5 (2083)
- Lymph & Longevity: The Untapped Secret to HealthFrom EverandLymph & Longevity: The Untapped Secret to HealthRating: 4.5 out of 5 stars4.5/5 (13)