Professional Documents
Culture Documents
Container and Closure
Uploaded by
DrGaurav TiwariOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Container and Closure
Uploaded by
DrGaurav TiwariCopyright:
Available Formats
Container and closure: Closures : Closures An effective closure must prevent the contents from escaping and allow
no substance to enter the container. The adequacy of the seal depends on Resiliency of the liner Flatness of the sealing surface on the container The tightness r torque with which it is applied. Five basic designs of closures: Five basic designs of closures Threaded screw cap Lug cap Crown cap Roll on closures Nonreusable roll-on closures Pilerproof closures 1. Threaded screw cap: 1. Threaded screw cap Threads engage with the corresponding threads on the neck of the container. Liners are pressed against the opening of the container that seals the product by overcoming sealing surface irregularities. Made of metal e.g tinplate or aluminium. Also made from plastics e.g. thermosetting and thermoplastic materials. Metal caps are coated inside with enamel or lacquer for resistance to corrosion. 2. Lug cap: 2. Lug cap Same as threaded screw cap but interrupted thread on glass finish instead of continuous thread. Engage a lug on the cap sidewall and sealed by pressing but requires quarter turn. Used for both normal atmospheric-pressure and vacuum-pressure closing. Widely used in food industry and also for parenteral equipments. 3. Crown cap (crimp on): 3. Crown cap (crimp on) Used for crimped closure for beverage bottles Remain unchanged for more than 50 years. 4. roll-on closures: 4. roll-on closures Made up from aluminum so sealed securely, opened easily and resealed effectively. Used for packaging of food, beverages, chemicals, and pharmaceutical. Resealable , non- resealable and pilerproof type roll-on closure are available for glass, plastic bottles and jars. Available in form of straight-sided, threadless shell After filling forms the threads on the packaging line. Allows for dimensional variation and fits a specific container. 5. Pilerproof closures: 5. Pilerproof closures Same as roll-on closure but greater skirt length. Additional length extends below the threaded portion to form a bank. Which fastened to the basic cap by a series of narrow metal bridges. When cap is removed bridges break and bank remains in place on the neck of the container. Non-reusable roll-on closures: Non-reusable roll-on closures Require unthreaded glass finishes. The skirts of these closures are rolled under retaining rings on the glass container. They are tear-off tabs that make them tamperproof and pilerproof. Closure liners: Closure liners A liner may be defined as any material that is inserted in a cap to effect a seal between the closure and the container. Made of resilient backing and facing material. Backing material must be soft enough to take up any irregularities in the sealing surface and elastic
enough to recover its original shape when removed and replaced. It is glued into cap with adhesive or cap can be made with undercut so free to rotate. Factors in selecting a liner: Factors in selecting a liner Chemically inert with product Gas and vapor transmission rate E.g of liner material Polyethylene Saran Aluminum foil Tinfoil Polyester Vinylite Yellow-oil Types of liner: Types of liner Torque testing: Torque testing The owens-illiois torque tester is used for tourque testing to check cap tightness on a packaging line because To prevent evaporation or leakage of the product Breakage of the plastic molded closure Application of a cap too tight to be removed. Rubber stoppers: Rubber stoppers To make stoppers, cap liners and bulbs for dropper assemblies. For multi-dose vials and disposable syringes. Rubber polymers are used e.g. natural, neoprene and butyl rubbers. Ingredient in rubber stoppers are Rubber, vulcanizing agent, accelerator/activator, extended filler, reinforced filler, softener/plasticizer, antioxidant, pigment, waxes. Complications associated with rubber stoppers: Complications associated with rubber stoppers Complex to manufacture May absorb active ingredient, antibacterial preservative from parenteral products Interfere with chemical analysis of the active ingredient Affect the toxicity or pyrogenicity of the injectable product Affect the chemical and physical stability of the preparation. Thermosetting resins: Thermosetting resins Phenolic and urea are mostly used. First soften under heat and then cures and hardens to a final state. Shaping must occur in first stage of softening. Once it is molded, no change in shape even upon reapplication of heat and pressure. Fabricated by compression molding. The manufacturing process is relatively slow but with better control and quick response to change in temperature and material flow. Phenolics : Phenolics Available in different grades and in dark colors (black and brown). Rigid, heat and chemical resistance and good strength. But color limitation. Withstand the torquing forces of capping machine and maintains a tight seal over a long period of time. Resistant to dilute acids and alkalies Strong alkalies decompose phenolics. Urea : Urea Hard translucent material takes coloring well. More expensive than phenolics . Heat resistance. Elegant colors are available Withstand high temperature. Absorb water under wet conditions but no serious effect on product. Not affected by organic acids but affected by alkalies and strong acids. Good resistance to all types of oils and greases Cant be steam sterilized. Thermoplastic resins: Thermoplastic resins Polystyrene, polyethylene and polypropylenes are mostly used. Each material have specific advantages depending on their physical and chemical properties desired for the application and on the particular product being packaged.
Classification of plastic packaging material and its physicochemical properties: Classification of plastic packaging material and its physicochemical properties Introduction: Introduction The word plastic is derived from the Greek word ( plastikos ) meaning capable of being shaped or molded. A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce costs. Types of plastic: Types of plastic Broadly devided into two categries which are as- Thermoplastics: - -it is mainly used in the injection molding, blow molding, extrusion and fabricated sheeting. Thermosets :-these plastic are used when good dimensional and temprature stabilty are required . Thermoplastics: Thermoplastics Acrylics -a hard thermoplastic made from acrylic acid or a derivative of acrylic acid. -low water absorption -fair tensile strenth -good electrical resistivity -heat resistance is low -used in hospital devices Nylon -Not one material but a group of very tough and flexible materials called polyamides. -good chemical resistance -used as a part and adapter of devices eg : aerosols valve -also used in manufacturing of laminates and films -have disadvantage that it wrinkled during autoclaving. Polyethylene:- -vary according to moleculer weight -low density or branched -high density or linear - linear type is more crystalline,heat resistantand stiffer. -both have low water absorption -excellent electrical resistance and chemical resistance -widely used as container for liquid and dry products Polysterene :- -one of the oldest and widely used plastic polymer - in pharma industry widely used for fabrication of container s and syringe. -low heat resistance and attacked by various chemical agents Ethylene vinyl acetate(EVA):- -it have good clarity -low temperature flexibility and toughness goood impact strength -less hardness Polypropylene:- -it is lighter than polyethylene -so it is much stiffer and heat resistant than polyethylene -widely used for solid dosage form products Polycarbonates:- -Form by condensation of polyphenols -polymer are transparent thermoplastics -have high strength and high temperature resistance Thermosets: Thermosets Phenol formaldehyde:- -provide good scratch resistant -low shrinkage and low water absorption Melamine formaldehyde:- -exhibit good resistance to oils, grease,and many organic solvents Urea formaldehyde:- -exhibit good dimensional stability -provide good resistance to alcohols,grease,and some weaker acids. Additives in plastic: Additives in plastic Lubricants :- -used to assist the processing of the plastic during the molding or extrusion operation. eg :- zinc stearate Stabilizer:- -used to prevent the degradation of the polymer by heat, light and chemicals. eg :-fatty acids salts, inorganic oxides Plasticizers: - -used to achieve flexiblity and softness. eg :- vinayls , cellulosics Antioxidants: - -used to prevent oxidation of the polymer. eg :- vitamin E
Antistatic agents:- -used to prevent the buildup of the static charge. Slip agents:- -used to reduce the coefficient of the friction of the matrial . Dyes and pigments :- -are used to impart colour . Physicochemical properties: Physicochemical properties Mass transfer:- - many pharmaceutical product must be protected from o 2, water vapour , co 2 and many other permeants for eg : and effervacent tablet requires a barrier from moisture. Permeation through a plastic barrier depends on the composition of plastic ,permeation area, thickness of the barrier and time. permeation through plastic also can be affected greatly by the addition of the additives and the crystalline property if the plastic. specific additives like plasticizers can increase the permeation rate greatly. highly crystalline plastic such as polypropylene generally exhibit low water permeation rates. Permeability rate of selective plastic materials:- Nylon water vapour permeability is 16-22 g/100in2/24 hr @37.5c Polyethylene water vapour permeability is 1-1.5 g/100in2/24 hr@37.5 Polysterene water c vapour permeability is 7-10 g/100in2/24 hr @37.5c Vinyl Oxygen permeability is 4-30 cc/100in2/mil/24hr/atm@25c Chemical attack: Chemical attack Resistance to acids, alkalies , fats, solvents, water and light are important if compatiblity with these materials is required. some plastics are incompatible with plasticizers used with PVC polymers, detergents and antiseptic solutions. absorption of the migrating chemical forces the polymer chain to apart , swells the plastic and cause stress cracking. For eg : rubber exposed to ozone will lose elasticity and become brittle. In the case of plastic used in the direct contact with the product in either dry or liquid form the lenth of the time that the medication and the container are in contact may determine if problems such as discolouration , leaching and absorption of a constituent of the product may arise. it is possible that both the product and the container it could change significantly from the time of manufacture . A specific storage condition also required to decrease the chemical attack of a product. Safety testing : Safety testing These test are performed to ensure the safety of use of any plastic. these are biological, physical, chemical and pharmacological assesments . The official compendia provide procedure for performing certain biological and physiological tests on plastic containers such as : Biological test:- use to determine the suitablity of the plastic materials intended for use in fabricating containers for pharmaceutical preprations . Physicochemical tests:- used to determine the physical and chemical properties of the plastics used as containers. Failure mode analysis: Failure mode analysis After development and subsquent use of plastic packaged items, functional problems may accur occasionally. Resolution of these problems require analysis of the causative failure mode.this involves problem isolation, segrating the problems material to a particular batch for eg :- to identify a potential causative factors. The failed parts are subjected to machanical , microscopic and chemical analysis for further determination of how they differ from acceptable parts. Physical tests such as machanical , electrical and optical determinations can be performed quickly and control values exist in the form of manufacturers specifications which are readily available. Chemical analysis of impurities also has done for failure mode analysis. PLASTIC CONTAINER FOR PHARMACEUTICALS:
PLASTIC CONTAINER FOR PHARMACEUTICALS Presented by Sachin Gawai Guided by Dr. Shivkanya fuloria Content : Content CLASSIFICATION OF PLASTIC, PHYSIOCHEMICAL MECHANICAL AND BIOLOGICAL PROPERTIES , PLASTIC POLYMER, ADDITIVES AND FABRICATION PROCESSES. PLASTIC CONTAINER FOR PHARMACEUTICALS: PLASTIC CONTAINER FOR PHARMACEUTICALS A plastic container for pharmaceutical use is a plastic article which contains or is intended to contain a pharmaceutical product and is, or may be, in direct contact with it. The closure is a part of the container. A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass , and may contain other substances to improve performance and/or reduce costs. Monomers of plastic are either natural or synthetic organic compounds. CLASSIFICATION OF PLASTIC: CLASSIFICATION OF PLASTIC There are two types of plastics: thermoplastics and thermosetting polymers . Thermoplastics are the plastics that don't undergo chemical change in their composition when heated and can be molded again and again; examples are polyethylene , polystyrene , polyvinyl chloride and polytetrafluoroethylene (PTFE). Thermosets can melt and take shape once; after they have solidified, they stay solid. The raw materials needed to make most plastics come from petroleum and natural gas . PHYSIOCHEMICAL MECHANICAL AND BIOLOGICAL PROPERTIES : PHYSIOCHEMICAL MECHANICAL AND BIOLOGICAL PROPERTIES 1)Mechanical properties of plastic:- Tensile strength Impact strength Tear strength Stiffness Coefficient of friction or slip Cont..: Cont.. Blocking Fatigue Creep failuer Hot tack Abrasion and Shock test 2)Optical properties: 2)Optical properties Light transmission Clarity Haze Gloss 4)PHYSIOCHEMICAL properties: 4)PHYSIOCHEMICAL properties Mass transfer Chemical attack Safety testing Failure model analysis PHYSIOCHEMICAL testing: PHYSIOCHEMICAL testing Non volatile residues Residue on ignition Heavy meta Buffering capacity and reaction Light transmission of plastic Moisture permeation Biological testing: Biological testing Pyrogenicty Blood compatibility Antigen city Suitability for used in CVS devices Gene and tissues toxicity reaction PowerPoint Presentation: ADVANTAGES OF PLASTICS:- Flexible and not easily broken. Low density and light in weight. Are cheap DISADVANTAGES OF PLASTICS:- They are not as chemically inert as Type -I
glass. They are not as impermeable to gas and vapour as glass. They may possess an electrostatic charge which will attract particles. PowerPoint Presentation: PLASTICS Polymer Many + Parts Latin: Plasticus, that which can be molded This name hints at how polymers are made This name honors plastics useful property of being easily molded PowerPoint Presentation: Polymer The word, polymer , implies that polymers are constructed from pieces ( monomers ) that can be easily connected into long chains ( polymer ). When you look at the above shapes, your mind should see that they could easily fit together. monomers PLASTIC POLYMERS: PLASTIC POLYMERS Thermoplastic Polyethylene Polypropylene Polystyrene Nylon Polycarbonate Polyethylene terep hthalate, etc. Polyvinyl chloride
PowerPoint Presentation: Thermosets Melamine formaldehyde Phenol formaldehyde Urea formaldehyde,etc. Polyethylene : Polyethylene A polymer made form just one monomer is polyethylene. There are two types of polyethylene polymers (plastics). LDPE AND HDPE It is a good against moisture, but relatively poor one against oxygen and other gases. Most solvent do not attack polyethylene. It is unaffected by the strong acid and alkalis. PowerPoint Presentation: These are the carbon atoms with their double-bond (2 shared electrons each). The hydrogen atoms are not shown. A collision breaks the first bond. Once the first double bond is broken, a chain reaction will occur. In about a second an entire chamber of compressed ethylene gas turns into the polymer, polyethylene. HDPE: HDPE High Density Polyethylene Used in milk, juice and water containers in order to take advantage of its protective barrier properties Its chemical resistance properties make it a good choice as container for household chemicals and detergents. Low-density polyethylene : Low-density polyethylene Low-density polyethylene ( LDPE ) is a thermoplastic made from petroleum . It was the first grade of polyethylene, produced in 1933 by Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization . Its manufacture employs the same method today. LDPE is commonly recycled and has the number "4" as its recycling symbol . PowerPoint Presentation: LDPE has more branching (on about 2% of the carbon atoms) than HDPE , so its intermolecular forces ( instantaneous-dipole induced-dipole attraction ) are weaker, its tensile strength is lower, and its resilience is higher. Also, since its molecules are less tightly packed and less crystalline because of the side branches, its density is lower. LDPE contains the chemical elements carbon and hydrogen . Chemical resistance :
Chemical resistance Excellent resistance (no attack) to dilute and concentrated acids , alcohols , bases and esters Good resistance (minor attack) to aldehydes , ketones and vegetable oils Limited resistance (moderate attack suitable for short-term use only) to aliphatic and aromatic hydrocarbons , mineral oils , and oxidizing agents Poor resistance, and not recommended for use with Halogenated hydrocarbons . Polyvinyl chloride : Polyvinyl chloride Polyvinyl chloride , ( IUPAC Poly(chloroethanediyl) ) commonly abbreviated PVC , is a thermoplastic polymer . It is a vinyl polymer constructed of repeating vinyl groups (ethenyls) having one of their hydrogens replaced with a chloride group. Polyvinyl chloride is the third most widely produced plastic , after polyethylene and polypropylene . PVC is widely used in construction because it is cheap, durable, and easy to assemble. PowerPoint Presentation: It can be made softer and more flexible by the addition of plasticizers , the most widely used being phthalates . Preparation Polyvinyl chloride is produced by polymerization of the vinyl chloride monomer (VCM), as shown. PowerPoint Presentation: Another polymer, which is almost the same as polyethylene, is P oly V inyl C hloride or PVC . The difference is that every other hydrogen is replaced with a chlorine atom ( green sphere). Properties : Properties Clarity Low cast Great fabrication flexibilty Fairly good oxygen barrier Greater stiffness Inexpensives Tough Degradation temp 280 0 c Cont.: Cont. Excellent barrier for oil, both volatile and fixed alcohols. And petroleum solvents Fairly good moisture barrier Not affected by acids or alkalies excepts for some oxydizing acids Impact resistance is poor at low temp PP: PP Polypropylene High tensile strength, ideal for caps and lids with threaded openings High melting point so can be hot-filled with products that then will cool Also used for products that need to be incubated, such as yogurt Chemical and physical properties : Chemical and physical properties Perfectly isotactic PP has a melting point of 171 C (340 F) Most commercial polypropylene is isotactic and has an intermediate level of crystallinity between that of low-density polyethylene ( LDPE ) and high-density polyethylene ( HDPE ). Polypropylene is normally tough and flexible, especially when copolymerized with ethylene. This allows polypropylene to be used as an engineering plastic . Polypropylene is reasonably economical, and can be made translucent Applications : Applications Many plastic items for medical or laboratory use can be made from polypropylene because it can withstand the heat in an autoclave . Its most common medical use is in the synthetic, nonabsorbable suture Prolene , manufactured by Ethicon Inc. PS:
PS Polystyrene In its crystalline form, it is a colorless plastic that can be clear and hard. Polystyrene can be recycled, and has the number "6" as its recycling symbol although the low cost of virgin polystyrene can be a discouragement. Polystyrene takes a very long time to biodegrade . Low in cost Polystyrene is not useful for liquid product Cont.: Cont. High water vapor transmission as well as high oxygen permeability Polystyrene container are easily scratched and often cracked when dropped Low melting point (190 0 F) Resistant to acid except strong oxidizing acids and to alkalies It is attacked by many chemicals which cause it to craze and crack and so it is generally used for packaging dry product only PowerPoint Presentation: Polystyrene is a thermoplastic substance, which is in solid (glassy) state at room temperature, but flows if heated above its glass transition temperature (for molding or extrusion), and becomes solid again when cooled. Pure solid polystyrene is a colorless, hard plastic with limited flexibility. It can be cast into molds with fine detail. Polystyrene can be transparent or can be made to take on various colors. Properties: Properties Density 1.05 g/cm 3 Density of EPS 16640 kg/m 3 Dielectric constant 2.42.7 Electrical conductivity (s) 10 16 S /m Thermal conductivity (k) 0.08 W/(mK) Young's modulus ( E ) 30003600 MPa Tensile strength ( s t ) 4660 MPa Elongation at break 34% Notch test 2 5 kJ /m 2 Glass transition temperature 95 C Melting point 240 C Vicat B 90 C Linear expansion coefficient (a) 810 5 / K Specific heat ( c ) 1.3 kJ/(kgK) Water absorption (ASTM) 0.030.1 Decomposition X years, still decaying Polycarbonate: Polycarbonate It is relatively expensive material Rigid Moderately chemical resistant Fair moisture barrier High impact strength Dimensional stability Resistance to strength Cont..: Cont.. Low water absorption, transparency and resistance to heat and flame Resistant to dilute acid , oxidizing or reducing agent, salt, oil, greases , and aliphatic hydrocarbon. Attacked by alkalies, amines, ketones, esters, aromatic hydrocarbon and some alcohol. Polycarbonate articles can be subjected to repeated sterilization in steam or water without undergoing significant degradation. PowerPoint Presentation: Polyethylene Terephthalate A clear, tough, polymer with exceptional gas and moisture barrier properties. PETs ability to contain carbon dioxide (carbonation) make it a good choice in soft drink bottles. Typically from by the reaction of terephthalic acid or dimethyl terephthalate with ethylene glycol in the presence of catalyst Excellent impact strength Excellent gas and aroma barrier Nylon (Polyamide): Nylon (Polyamide) Nylon is made from a dibasic acid combined with a diamine Since there are many dibasic acids and many different amines , there is great variety of nylons. The type of acid and amines that is used is indicated by an identifying number, thus nylon 6/10 has 6 carbon atoms in the diamine and in the acid PowerPoint Presentation:
It can be autoclaved and is extremely strong Quite difficult to destroy by mechanical means Resistant to wide range of organic and inorganic chemicals Highly impermeable to oxygen It is not good barrier to water vapor Relative high water transmission rate and possibility of drug plastic interaction FDA- cleared- nylon 6/6, nylon6, nylon 6/10, nylon 11 Thermosets : Thermosets These plastics are used when good dimension and temperature stability are required. Parts are fabricated by means of compression molding techniques The formaldehyde plastics are obtained by condensation reaction between formaldehyde and substances such as melamine , phenol , urea. Formaldehyde used in the pharmaceutical industry as a closure for glass and or plastic container. High resistance to heat, they are used in specific application where the molded parts require sterilization by steam. Urea formaldehyde: Urea formaldehyde Good dimensional stability Good strength Highly rigid Good resistance to alcohols , oils, grease, and some weaker acids. These properties permit use for injectionmolded heads for collapsible tubes used to contain liquid based topical product. Additives in Plastics: Additives in Plastics Additives are added to polymers in order to obtain or improve certain properties such as strength, stiffness, color, resistance to weather and flammability. Plasticizers are added to obtain flexibility and softness, most common use of plasticizers are in PVC. PowerPoint Presentation: Ultraviolet radiation (sunlight) and oxygen cause polymers to become stiff and brittle, they weaken and break the primary bonds. A typical treatment is to add carbon black (soot) to the polymer, it absorbs radiation. Antioxidants are also added to protect against degradation. Fillers such as fine saw dust, silica flour, calcium carbide are added to reduce the cost and to increase harness, strength, toughness, dimensional stability. PowerPoint Presentation: Colorants are added to obtain a variety of colors. Colorants are either organic (dye) or inorganic (pigments). Pigments provide greater resistance to temperature and sunlight. Flame retardants such as chlorine, phosphorus and bromine, are added to reduce polymer flammability. Teflon does not burn and nylon and vinyl chloride are self-extinguishing. Lubricants such as mineral oil and waxes are added to reduce friction. Manufacturing of plastic: Manufacturing of plastic Injection molding Extrusion Blow molding Solvent casting Compression molding References : References Lippincott Williams And Wilkins, ,Remington, the science and practice of pharmacy, volume 1, 21 st ,edition, published by Wolter kiuwer Health Pvt. Ltd ., New Delhi,pp.1047-1055. Leon Lachman, Herbert AL, Joseph LK., The theory and practice of industrial pharmacy, 3rd ed. Bombay: Varghese publishing house; 1990. p.p.711-733.
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Cosmeceutic ALS: Cosmetics and PharmaceuticalsDocument19 pagesCosmeceutic ALS: Cosmetics and PharmaceuticalssowjanyaNo ratings yet
- Crop Sci 1 Lecture Manual OverviewDocument86 pagesCrop Sci 1 Lecture Manual OverviewAnalYn Litawan Bucasan100% (1)
- Poultry Science MCQDocument12 pagesPoultry Science MCQelanthamizhmaran83% (6)
- Introspect For DealersDocument13 pagesIntrospect For DealersOBERON-INTROSPECT-BIOSPECTNo ratings yet
- Lec.6.Varietal & Hybrid Seed Production in RiceDocument75 pagesLec.6.Varietal & Hybrid Seed Production in RiceAmirthalingam KamarajNo ratings yet
- PrescriptionDocument30 pagesPrescriptionDrGaurav TiwariNo ratings yet
- Bio-Compatible and Bio - Degradable PolymersDocument29 pagesBio-Compatible and Bio - Degradable PolymersDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Pharmaceutical Sales Training Excellence: Tools, Processes & Resources That Drive EffectivenessDocument17 pagesPharmaceutical Sales Training Excellence: Tools, Processes & Resources That Drive EffectivenessDrGaurav TiwariNo ratings yet
- Drug Release Kinetics ModelsDocument7 pagesDrug Release Kinetics ModelsSajid Khan SadozaiNo ratings yet
- Mpharma PharmaceuticsDocument14 pagesMpharma PharmaceuticsDrGaurav TiwariNo ratings yet
- Important DocumentDocument2 pagesImportant DocumentDrGaurav TiwariNo ratings yet
- Article Wjpps 1386011514Document7 pagesArticle Wjpps 1386011514Donig FermanianNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- 2009 Drug InformationDocument4 pages2009 Drug InformationXee JayNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Radio PharmaceuticsDocument52 pagesRadio PharmaceuticsDrGaurav TiwariNo ratings yet
- Radio PharmaceuticsDocument52 pagesRadio PharmaceuticsDrGaurav TiwariNo ratings yet
- Management of IPR in IndiaDocument12 pagesManagement of IPR in IndiaDrGaurav TiwariNo ratings yet
- Metronidazole-Loaded Bioabsorbable Films As Local Antibacterial Treatment of Infected Periodontal PocketsDocument8 pagesMetronidazole-Loaded Bioabsorbable Films As Local Antibacterial Treatment of Infected Periodontal PocketsDrGaurav TiwariNo ratings yet
- Management of IPR in IndiaDocument12 pagesManagement of IPR in IndiaDrGaurav TiwariNo ratings yet
- Uppsc Fee CollectionDocument2 pagesUppsc Fee CollectionDrGaurav TiwariNo ratings yet
- Automation in Pharmaceutical ManufacturingDocument6 pagesAutomation in Pharmaceutical ManufacturingDrGaurav TiwariNo ratings yet
- 1st Middle East Six Sigma ForumDocument29 pages1st Middle East Six Sigma ForumanandweNo ratings yet
- Bhavya TQMDocument34 pagesBhavya TQMDrGaurav TiwariNo ratings yet
- QaDocument3 pagesQaDrGaurav TiwariNo ratings yet
- Application Form-PGTD TEACHERS 05 Feb 2013Document8 pagesApplication Form-PGTD TEACHERS 05 Feb 2013DrGaurav TiwariNo ratings yet
- ImplantsDocument2 pagesImplantsDrGaurav TiwariNo ratings yet
- Application Form-PGTD TEACHERS 05 Feb 2013Document8 pagesApplication Form-PGTD TEACHERS 05 Feb 2013DrGaurav TiwariNo ratings yet
- CopyrightDocument27 pagesCopyrightDrGaurav Tiwari0% (1)
- IN SEARCH OF SEXUAL ENHANCEMENT THROUGH PILLS AND DRUGSDocument16 pagesIN SEARCH OF SEXUAL ENHANCEMENT THROUGH PILLS AND DRUGSJakob AndradeNo ratings yet
- SOP EcoliDocument3 pagesSOP EcoliIeqa HaziqahNo ratings yet
- TC QMM 56942Document120 pagesTC QMM 56942Fernando R EpilNo ratings yet
- Biomedical Physics, Vol 7 @medphyslibDocument360 pagesBiomedical Physics, Vol 7 @medphyslibStats -MANNo ratings yet
- Pengantar Mikrobiologi LingkunganDocument28 pagesPengantar Mikrobiologi LingkunganAhmad SyahrulNo ratings yet
- Determination of Lethal Dose Ld50of Venom of Four Different Poisonous Snakes Found in PakistanDocument4 pagesDetermination of Lethal Dose Ld50of Venom of Four Different Poisonous Snakes Found in PakistanSutirtho MukherjiNo ratings yet
- The Antibacterial Properties of Isothiocyanates PDFDocument15 pagesThe Antibacterial Properties of Isothiocyanates PDFSasicha DoungsuwanNo ratings yet
- RT-PCR Kit for RNA Detection up to 6.5kbDocument14 pagesRT-PCR Kit for RNA Detection up to 6.5kbLatifa Putri FajrNo ratings yet
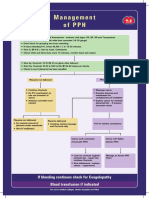
- Management of PPHDocument1 pageManagement of PPH098 U.KARTHIK SARAVANA KANTHNo ratings yet
- The "Five Families" College Essay ExampleDocument1 pageThe "Five Families" College Essay ExampleKishor RaiNo ratings yet
- DNA TimelineDocument2 pagesDNA TimelineMinaMilićNo ratings yet
- 30395-Article Text-56938-1-10-20220916Document8 pages30395-Article Text-56938-1-10-20220916Djim KARYOMNo ratings yet
- Perkecambahan Dan Pertumbuhan Palem Jepang (Actinophloeus Macarthurii Becc.) Akibat Perendaman Biji Dalam LumpurDocument7 pagesPerkecambahan Dan Pertumbuhan Palem Jepang (Actinophloeus Macarthurii Becc.) Akibat Perendaman Biji Dalam LumpurNurma YahyaNo ratings yet
- Transport Phenomena in Nervous System PDFDocument538 pagesTransport Phenomena in Nervous System PDFUdayanidhi RNo ratings yet
- Uits PDFDocument36 pagesUits PDFCrystal ParkerNo ratings yet
- General Katalog PT. AmoebaDocument23 pagesGeneral Katalog PT. AmoebaMulyanaNo ratings yet
- Cellular RespirationDocument20 pagesCellular RespirationAlessiaNo ratings yet
- Noscapine - British PharmacopoeiaDocument3 pagesNoscapine - British PharmacopoeiaSocial Service (V)No ratings yet
- 皮膚病介紹Document93 pages皮膚病介紹MK CameraNo ratings yet
- Thrombolytic Therapy in Emergency MedicineDocument9 pagesThrombolytic Therapy in Emergency MedicinemedeviNo ratings yet
- Short Periods of Incubation During Egg Storage - SPIDESDocument11 pagesShort Periods of Incubation During Egg Storage - SPIDESPravin SamyNo ratings yet
- Amazing Facts - Exercises 1Document3 pagesAmazing Facts - Exercises 1Alina DorobatNo ratings yet
- Self Concept Inventory Hand OutDocument2 pagesSelf Concept Inventory Hand OutHarold LowryNo ratings yet
- MR Spectroscopy: Technical Aspects and Clinical ApplicationsDocument5 pagesMR Spectroscopy: Technical Aspects and Clinical ApplicationsNotariana Kusuma ANo ratings yet
- Chapter 2 Medical Terminology Verified AnswersDocument5 pagesChapter 2 Medical Terminology Verified AnswersGregg ProducerNo ratings yet