Professional Documents
Culture Documents
Electrochemistry
Uploaded by
danielmahsaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemistry
Uploaded by
danielmahsaCopyright:
Available Formats
Electrochemistry
Electrochemistry is the study of the relationship between chemical reactions and electrical work. Galvanic cells are cells where chemical reactions give rise to electrical energy. Electrolytic cells are cells where a supply of electrical energy is converted into chemical energy. Galvanic cells chemical energy electrical energy When a metal is immersed in a solution of its ions, two possible electron transfer reactions can take place:
At equilibrium, there is a potential difference between the metal and an aqueous solution of its ions called its electrode potential. This arrangement is called a half-cell. The electrode potential of a metal depends on its : -temperature and pressure -nature of the metal -concentration of its ions. The absolute electrode potential of a metal cannot be measured. We have to compare the previous half-cell to another half-cell , called the hydrogen half-cell, and measure the potential difference between the two half-cells using a voltmeter. A standard hydrogen electrode or standard hydrogen half-cell is the half-cell represented by the equation:
Under standard conditions. Standard conditions refer to: Temperature of Pressure of any gas involved is Concentration of any ion involved is The standard hydrogen electrode consists of a platinum electrode dipped into H+ ions with pure H2 gas bubbling over it under a pressure of 1 atm and at 298K. 1
Arbitrarily, the standard electrode potential for this system E (H+/H2) is assigned a value of The standard electrode potential (E ) of a half-cell is the potential difference between this half-cell and the standard hydrogen electrode, measured under standard conditions.
To measure the standard electrode potential of Zn The standard zinc half-cell is connected to the standard hydrogen half-cell by a salt bridge, which contains K+NO3- ions. The salt-bridge completes the circuit by allowing the passage of charged ions between electrodes but physically separating the two half-cells. There is a voltmeter. The cell registers its max. Potential difference, which is called the emf of the cell. In this case, Ecell at 298K is found to be 0.76V. The standard electrode potential E , for Zn2+/Zn is -0.76V. The reactions at the electrodes are : oxidation Reduction Giving an overall cell reaction:
When an electrode is connected to the standard hydrogen electrode: E >0, reduction is taking place at the electrode. E <0, oxidation takes place at the electrode. On the left hand side of the equation, it is the placement of the oxidised species and the electrons, on the right hand side of the equation, it is the reduced species. Electrons are on the left. The electrochemical series is also called the redox potential series. It is an activity series in which the metals are placed in order of increasing electrode potential. The more reactive metals are placed higher in the series. The more reactive the metal, the greater is its tendency to react and to form positive ions, therefore more negative E value. A large positive value of E indicates a high likelihood of reduction, a high likelihood of the above reaction proceeding from left to right. A large negative value of E indicates a high likelihood of oxidation, a high likelihood of the above reaction proceeding from right to left. 2
If a half-equation is multiplied by a number, E value stays the same. If you reverse a half-equation, the E value is inverted. A galvanic cell showing a Zn2+/Zn half-cell connected to a Cu2+/Cu half-cell.
Data :
When given two reduction half-reactions, the reduction half-reaction with the more positive E always takes place as written as a reduction, while the other half-reaction is forced to run in reverse as an oxidation.
Oxidation : Zn Reduction : Cu2+ + 2 e Overall cell reaction: Anode: Zn Cathode : Cu Anode is where oxidation occurs, cathode is the electrode where reduction occurs. Zn anode : negatively charged. Cu electrode : positively charged. E cell = Ereduction Eoxidation = +0.34 (-0.76) = +1.10V The reaction is feasible because Ecell >0. The electrons flow from the zinc anode via the external wire to the Cu cathode. The salt bridge: An electrolyte is a liquid that conducts electricity as a result of the presence of positive and/or negative ions. Usually aqueous ionic solutions. Strong electrolytes, weak electrolytes and non-electrolytes. Given 2 reactants, use E to predict redox reaction.
If Ecell >0, redox reaction is feasible or spontaneous. If Ecell <0, redox reaction is not feasible or non spontaneous.
Limitations of Ecell. Energetic vs kinetics We can use Ecell to predict whether a redox reaction is energetically feasible (delta G <0) but that does not give any information about the rate of reaction. Many reactions could have a high activation energy. Eg. H2 bubbled into Cu2+ ions. Ecell is feasible , but in reality no reaction occurs. We say that the reaction is energetically feasible but kinetically unfavourable. E values relate only to standard conditions. Changes in temp, pressure and concentration will affect the values of electrode potentials. We can use Le Chateliers principle to predict these changes. Practical applications of galvanic cells batteries and fuel cells. Batteries Zn (anode), MnO2 (cathode) Zn (anode), Ag2O (cathode) Rechargeable lead acid battery. Pb (anode), PbO2 (cathode) Fuel cell The electrode reactants in a fuel cell are supplied continuously and the cell is able to operate without theoretical limit as long as you supply reactants. Hydrogen-oxygen fuel cell:
H2 (anode) O2 (cathode) Alkaline electrolyte Advantages of fuel cells: No pollution They convert about 75% of the chemical energy of the fuel into useable power. Low maintenance cost as there is no moving parts to replace. 4
Disadvantages of fuel cells: Electrode reactions are slow. For cars, hydrogen must be transported either in cylinders or at very low temperature as a liquid. Bulky and expensive. Expensive Alkali may absorb carbon dioxide form air to form carbonates that clog up the porous electrodes. Electric cars: High mass of batteries Short driving ranges Low recharging rates High replacement costs of batteries.
Alternative to this fuel cell methanol fuel cell.
Copy out the two half-equations. Multiply the half-equations by appropriate factors so that the number of electrons involved in each is the same. Add the two half-equations together, cancelling out any ions or molecules that appear on both sides of the equation.
You might also like
- Introduction To Biopotential Measurement and Excitation: (Bioelectromagnetism - Jakko Malmivuo, Robert Plonsey)Document23 pagesIntroduction To Biopotential Measurement and Excitation: (Bioelectromagnetism - Jakko Malmivuo, Robert Plonsey)Lynn WankharNo ratings yet
- Participate in Board Questions: Ascexam / ReasceDocument105 pagesParticipate in Board Questions: Ascexam / ReasceSharma RomeoNo ratings yet
- Memorias FloydDocument36 pagesMemorias FloydDr.R.Udaiya Kumar Professor & Head/ECENo ratings yet
- Ecg 3Document10 pagesEcg 3Khalid KhassawnehNo ratings yet
- Design and Analysis of A Dual Chamber Cardiac Pacemaker Using VHDL in Biomedical ApplicationDocument3 pagesDesign and Analysis of A Dual Chamber Cardiac Pacemaker Using VHDL in Biomedical ApplicationEditor IJRITCCNo ratings yet
- Cardiac Defibrillation Mechanisms Challenges and Implications PDFDocument260 pagesCardiac Defibrillation Mechanisms Challenges and Implications PDFdr9348345000100% (1)
- Pacemaker Output Circuit Design for Optimal Patient StimulationDocument30 pagesPacemaker Output Circuit Design for Optimal Patient StimulationVishwanath ShervegarNo ratings yet
- Due: Monday September 17: Homework 2 - Solution ECE 445 Biomedical Instrumentation, Fall 2012Document3 pagesDue: Monday September 17: Homework 2 - Solution ECE 445 Biomedical Instrumentation, Fall 2012amastasia salsaNo ratings yet
- Biopotential ElectrodesDocument35 pagesBiopotential ElectrodesakshayNo ratings yet
- Unit 1 - Cardiac Care UnitsDocument29 pagesUnit 1 - Cardiac Care UnitssitalekshmiNo ratings yet
- Pacemaker Sense AmplifiersDocument48 pagesPacemaker Sense AmplifiersArbab Masood AhmadNo ratings yet
- Microprocessor in PacemakerDocument5 pagesMicroprocessor in PacemakerAbdullah JavaidNo ratings yet
- CXR Interpretation GuideDocument147 pagesCXR Interpretation GuideFcsieka FcseikaNo ratings yet
- Study Frequency CounterDocument8 pagesStudy Frequency CounterMahadevNo ratings yet
- Biomedical Engineering Theory and Practice-Biomedical Instrumentation-ElectrocardiographyDocument4 pagesBiomedical Engineering Theory and Practice-Biomedical Instrumentation-Electrocardiographyvicky_ani1986No ratings yet
- The Artificial Heart: A Design Example: BIOE 1000 October 18, 2001Document17 pagesThe Artificial Heart: A Design Example: BIOE 1000 October 18, 2001Paspulati Leelaram100% (1)
- Slyt416 Ecg EegDocument18 pagesSlyt416 Ecg EegsakthyinNo ratings yet
- Pulseless Pumps & Artificial HeartsDocument23 pagesPulseless Pumps & Artificial HeartscafemedNo ratings yet
- Implementation of Low Delay Dual Chamber Pacemaker Using VerilogDocument4 pagesImplementation of Low Delay Dual Chamber Pacemaker Using VerilogMeghanand KumarNo ratings yet
- Lecure-3 Biopotential ElectrodesDocument43 pagesLecure-3 Biopotential ElectrodesNoor AhmedNo ratings yet
- Electronics II NotesDocument41 pagesElectronics II NotesactuatorNo ratings yet
- ECG ImportantDocument82 pagesECG ImportantAyyoob JafariNo ratings yet
- Cardiovascular SystemDocument40 pagesCardiovascular SystemDouglas Jacques100% (1)
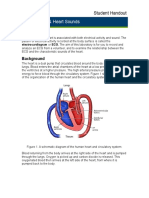
- ECG & Heart Sounds Lab ExperimentDocument5 pagesECG & Heart Sounds Lab ExperimentWalaa AlzoubiNo ratings yet
- 5 - Mastering Augmented Unipolar Limb LeadsDocument4 pages5 - Mastering Augmented Unipolar Limb LeadsSharan MurugaboopathyNo ratings yet
- ECG & Heart Sounds: Student HandoutDocument7 pagesECG & Heart Sounds: Student HandoutROHITNo ratings yet
- Chest X-RayDocument101 pagesChest X-RayYuke04No ratings yet
- Standard and Isolation PrecautionDocument31 pagesStandard and Isolation PrecautionMarriam LegazpiNo ratings yet
- Systolic Function 2Document6 pagesSystolic Function 2FlorenceLorenzoNo ratings yet
- IMD - Step-Up To USMLE Step 3 - Chapter 1 - CardiologyDocument124 pagesIMD - Step-Up To USMLE Step 3 - Chapter 1 - CardiologyAly SherifNo ratings yet
- Lecure-5 The Origin of Biopotentials - 2Document34 pagesLecure-5 The Origin of Biopotentials - 2Noor Ahmed100% (1)
- Austin Journal of Clinical CardiologyDocument15 pagesAustin Journal of Clinical CardiologyAustin Publishing GroupNo ratings yet
- 12-Lead ECG Monitoring With EASI PDFDocument14 pages12-Lead ECG Monitoring With EASI PDFalghashm001No ratings yet
- Lec 01-Introduction To Biomedical Signal ProcessingDocument35 pagesLec 01-Introduction To Biomedical Signal ProcessingfarsunNo ratings yet
- Heart Notes PDFDocument2 pagesHeart Notes PDFChris_Barber09No ratings yet
- MedicalelectricalsafetyDocument89 pagesMedicalelectricalsafetyMeruva LokeshwarNo ratings yet
- Simple Office SpirometryDocument42 pagesSimple Office SpirometryRoberto Merza III100% (4)
- Martin (2015) - Physics of UltrasoundDocument4 pagesMartin (2015) - Physics of Ultrasoundgatorfan786No ratings yet
- Medical Physics Ultrasound ImagingDocument17 pagesMedical Physics Ultrasound ImagingAvinash BoodhooNo ratings yet
- Medical ImagingDocument93 pagesMedical ImagingpraveenNo ratings yet
- Blood Gas AnalyzerDocument13 pagesBlood Gas Analyzeranon_708486566No ratings yet
- ADC Lecture1Document50 pagesADC Lecture1GaneshkumarmuthurajNo ratings yet
- UNIT 1 Biomedical EngineeringDocument128 pagesUNIT 1 Biomedical EngineeringgajulasureshNo ratings yet
- Band Theory of SolidsDocument26 pagesBand Theory of SolidsDizney Lobaton EsparteroNo ratings yet
- Measuring Biopotentials and ElectrophysiologyDocument30 pagesMeasuring Biopotentials and ElectrophysiologyFidaa Jaafrah100% (1)
- CARDIAC ELECTROPHYSIOLOGY AND THE ELECTROCARDIOGRAM - ClinicalKey PDFDocument40 pagesCARDIAC ELECTROPHYSIOLOGY AND THE ELECTROCARDIOGRAM - ClinicalKey PDFMelanie DascăluNo ratings yet
- DR Yassir Javaid Interpreting The 12 Lead ECGDocument28 pagesDR Yassir Javaid Interpreting The 12 Lead ECGTirtha TaposhNo ratings yet
- Ecg Treadmill and Holter TestDocument77 pagesEcg Treadmill and Holter TestRiteka Singh100% (1)
- Bio Accoustic 131203Document10 pagesBio Accoustic 131203Choirun Nisa Nur AiniNo ratings yet
- Unit 2: Biosignal Characteristics and Electrode ConfigurationsDocument34 pagesUnit 2: Biosignal Characteristics and Electrode ConfigurationsEZHIL NNo ratings yet
- Edc PDFDocument173 pagesEdc PDFsivaNo ratings yet
- Chapter 2 (3) PNP JunctionDocument7 pagesChapter 2 (3) PNP JunctionJoel WatsonNo ratings yet
- Fundamentals of Logic Design 6th Edition Chapter 13Document8 pagesFundamentals of Logic Design 6th Edition Chapter 13Huy HoangNo ratings yet
- The Radiation Chemistry of MacromoleculesFrom EverandThe Radiation Chemistry of MacromoleculesMalcolm DoleNo ratings yet
- Fundamentals of Microwave Electronics: International Series of Monographs on Electronics and InstrumentationFrom EverandFundamentals of Microwave Electronics: International Series of Monographs on Electronics and InstrumentationNo ratings yet
- Intelligence-Based Cardiology and Cardiac Surgery: Artificial Intelligence and Human Cognition in Cardiovascular MedicineFrom EverandIntelligence-Based Cardiology and Cardiac Surgery: Artificial Intelligence and Human Cognition in Cardiovascular MedicineNo ratings yet
- Men of Physics Lord Rayleigh–The Man and His Work: The Commonwealth and International Library: Selected Readings in PhysicsFrom EverandMen of Physics Lord Rayleigh–The Man and His Work: The Commonwealth and International Library: Selected Readings in PhysicsNo ratings yet
- Quiz #2 Agents, Spontaneous Reactions, Oxidation #'S, and StrengthDocument1 pageQuiz #2 Agents, Spontaneous Reactions, Oxidation #'S, and Strengthdanielmahsa0% (1)
- Calculation Involving Titration Part 2Document3 pagesCalculation Involving Titration Part 2danielmahsaNo ratings yet
- Organic Questions HomeDocument32 pagesOrganic Questions HomedanielmahsaNo ratings yet
- Chemistry Matter 130623221721 Phpapp01Document18 pagesChemistry Matter 130623221721 Phpapp01danielmahsaNo ratings yet
- Ideal Gas Equation PresentationDocument6 pagesIdeal Gas Equation PresentationdanielmahsaNo ratings yet
- 2017 Chemistry Trial Exam 1 Mark SchemeDocument1 page2017 Chemistry Trial Exam 1 Mark SchemedanielmahsaNo ratings yet
- Trial Exam Paper 1 April 2017Document20 pagesTrial Exam Paper 1 April 2017danielmahsaNo ratings yet
- OCR A2 Chemistry Student Teacher Technician Worksheets Activity 5Document3 pagesOCR A2 Chemistry Student Teacher Technician Worksheets Activity 5danielmahsaNo ratings yet
- AS Chemistry CIE Tutorial On Redox Reactions Set 1Document2 pagesAS Chemistry CIE Tutorial On Redox Reactions Set 1danielmahsaNo ratings yet
- Titration Questions Set 1Document8 pagesTitration Questions Set 1danielmahsaNo ratings yet
- Lattice Energy CIE Chemistry A2 Chemical EnergeticsDocument2 pagesLattice Energy CIE Chemistry A2 Chemical EnergeticsdanielmahsaNo ratings yet
- As Chemistry CIE Tutorial On Redox Reactions SET 3Document3 pagesAs Chemistry CIE Tutorial On Redox Reactions SET 3danielmahsaNo ratings yet
- Ionic Equilibria pH Calculations Buffers TitrationsDocument20 pagesIonic Equilibria pH Calculations Buffers TitrationsdanielmahsaNo ratings yet
- Ch5 Student NotesDocument69 pagesCh5 Student NotesdanielmahsaNo ratings yet
- Chapter 1 Question Set 2Document2 pagesChapter 1 Question Set 2danielmahsaNo ratings yet
- Chapter 1 Question Set 1Document2 pagesChapter 1 Question Set 1danielmahsaNo ratings yet
- As Chemistry QuestionDocument4 pagesAs Chemistry QuestiondanielmahsaNo ratings yet
- AS Chemistry CIE Tutorial: Atomic Structure Key ConceptsDocument3 pagesAS Chemistry CIE Tutorial: Atomic Structure Key ConceptsdanielmahsaNo ratings yet
- AS Chem Ionic Equilibria Set 3 Question 5Document2 pagesAS Chem Ionic Equilibria Set 3 Question 5danielmahsaNo ratings yet
- As Chem Ionic Equilibria Set 2question 4Document2 pagesAs Chem Ionic Equilibria Set 2question 4danielmahsaNo ratings yet
- Ionisation EnergiesDocument3 pagesIonisation EnergiesdanielmahsaNo ratings yet
- As Chemistry Tutorial Ionic EquilibriaDocument6 pagesAs Chemistry Tutorial Ionic EquilibriadanielmahsaNo ratings yet
- Isotopes of Iron in Meteorite Sample AnalyzedDocument3 pagesIsotopes of Iron in Meteorite Sample AnalyzeddanielmahsaNo ratings yet
- As Chem Ionic Equilibria Set 4 Question 6Document2 pagesAs Chem Ionic Equilibria Set 4 Question 6danielmahsaNo ratings yet
- As Chemistry Tutorial Chemical Equilibria Set 1Document2 pagesAs Chemistry Tutorial Chemical Equilibria Set 1danielmahsaNo ratings yet
- As Question 4Document3 pagesAs Question 4danielmahsaNo ratings yet
- Structure Determination Using IR SpectrosDocument8 pagesStructure Determination Using IR SpectrosdanielmahsaNo ratings yet
- Electronic Configuration GuideDoc2Document1 pageElectronic Configuration GuideDoc2danielmahsaNo ratings yet
- As Question 6Document1 pageAs Question 6danielmahsaNo ratings yet
- As Chem Atomic Structure Question 2Document1 pageAs Chem Atomic Structure Question 2danielmahsaNo ratings yet
- Electrochem. Solid State Lett. 2002 Chae C64 6Document3 pagesElectrochem. Solid State Lett. 2002 Chae C64 6Anonymous SIG8xwx9ANo ratings yet
- Chemical Equilibrium Worksheet 1 AnswersDocument2 pagesChemical Equilibrium Worksheet 1 AnswersYing Liang33% (3)
- Urriculum: AS Level ChemistryDocument18 pagesUrriculum: AS Level ChemistrythegreatwardiniNo ratings yet
- Effect of Trace O2 on Catalyst Dispersion AnalysisDocument7 pagesEffect of Trace O2 on Catalyst Dispersion Analysismanager's hubNo ratings yet
- 2021, Understanding Spatial Effects of Tetrahedral and Octahedral Cobalt CationsDocument12 pages2021, Understanding Spatial Effects of Tetrahedral and Octahedral Cobalt CationsTRINH HUỲNH NGỌC DIỄMNo ratings yet
- Cathodic Protection Afforded by An Intermittent Current Applied To Reinforced ConcreteDocument21 pagesCathodic Protection Afforded by An Intermittent Current Applied To Reinforced ConcreteErol DAĞNo ratings yet
- 18 NTU TE 0060 - PDFDocument10 pages18 NTU TE 0060 - PDFFahad HussainNo ratings yet
- Muravha, E. (2012)Document19 pagesMuravha, E. (2012)WilliamsRafaelMataRimacNo ratings yet
- Part 2 Physical ScienceDocument5 pagesPart 2 Physical ScienceRonald A. CarniceNo ratings yet
- Design of Copper Solvent Extraction Circuit - Effect of Dithionate On Copper Solvent Extraction RecoveryDocument148 pagesDesign of Copper Solvent Extraction Circuit - Effect of Dithionate On Copper Solvent Extraction Recoveryjoseph kafumbilaNo ratings yet
- Application Chemical Kinetics To Deterioration of Foods - 1-6Document6 pagesApplication Chemical Kinetics To Deterioration of Foods - 1-6Cuervo CuervoNo ratings yet
- SOALAN STRUKTUR Form 5Document9 pagesSOALAN STRUKTUR Form 5Willey TaluanNo ratings yet
- Photocatalytic Degradation of P-Nitrophenol in An Annular Column Photoreactor and The IntermediatesDocument7 pagesPhotocatalytic Degradation of P-Nitrophenol in An Annular Column Photoreactor and The Intermediatessulihah12No ratings yet
- Chemical Reactions and EquationsDocument2 pagesChemical Reactions and Equationsnarayana sapNo ratings yet
- Deep Eutectic Solvents For Cathode Recycling PDFDocument7 pagesDeep Eutectic Solvents For Cathode Recycling PDFalenmeNo ratings yet
- Drug Quality ControlDocument20 pagesDrug Quality Controlprinz1mendezNo ratings yet
- Corrosion Course ActivityDocument13 pagesCorrosion Course Activitymanjunath koraddiNo ratings yet
- A Study On Oxide Scale Formation of Low Carbon SteelDocument12 pagesA Study On Oxide Scale Formation of Low Carbon SteelDebora ChavezNo ratings yet
- Igcse (9-1) Edexcel - ChemistryDocument9 pagesIgcse (9-1) Edexcel - ChemistryTajryan TanimNo ratings yet
- JC2 Chemistry H2 2018 MeridianDocument109 pagesJC2 Chemistry H2 2018 MeridianYao Le Titanium ChenNo ratings yet
- Government Engineering College Bhuj: Gujarat Technological UniversityDocument23 pagesGovernment Engineering College Bhuj: Gujarat Technological UniversityPâtël PrãtïkNo ratings yet
- IIT-Foundation-Olympiad-Explorer Class 8 Chemistry PDFDocument29 pagesIIT-Foundation-Olympiad-Explorer Class 8 Chemistry PDFShikhar60% (10)
- 0653 m18 QP 22Document16 pages0653 m18 QP 22Thao TrinhNo ratings yet
- Versatile Methods for Benzimidazole SynthesisDocument13 pagesVersatile Methods for Benzimidazole SynthesisLe RonoNo ratings yet
- 0705-Enthusiast Score-II - (TEAS, T-AS, TOAS, TNAS, TRAS & TMAS) - Hs.Document19 pages0705-Enthusiast Score-II - (TEAS, T-AS, TOAS, TNAS, TRAS & TMAS) - Hs.Prashant BhattNo ratings yet
- GRB Physical Chemistry IIT JEE PDFDocument995 pagesGRB Physical Chemistry IIT JEE PDFsachin gupta75% (12)
- Analysis of SPM Chemistry Paper 3Document3 pagesAnalysis of SPM Chemistry Paper 3Luk HKNo ratings yet
- Das Et Al., 2018Document7 pagesDas Et Al., 2018ASTRID BARCO TULANDENo ratings yet
- Acid and Base Study Guide Multiple Choice QuestionsDocument3 pagesAcid and Base Study Guide Multiple Choice Questionsسلمان الحربيNo ratings yet