Professional Documents
Culture Documents
Dr. Shubhangi Mhaske - OSF
Uploaded by
Asma NawazCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dr. Shubhangi Mhaske - OSF
Uploaded by
Asma NawazCopyright:
Available Formats
Review Article
Oral submucous fibrosis - Current Concepts in Etiopathogenesis
M.K. Gupta*, Shubhangi Mhaske, Raju Ragavendra , Imtiyaz
Department of Oral & Maxillofacial Surgery*, Department of Oral & Maxillofacial Pathology, Peoples Dental Academy, Bhanpur, Bhopal-462010 (M.P.)
Abstract:
Oral submucous fibrosis (OSF) is now accepted globally as an Indian disease, having highest malignant potential than any other oral premalignant lesions. The understanding of the exact role of alkaloids and other etiological agents with respect to pathogenesis will help the management and minimize the blind clinical trials and treatment modalities. This article provides an overview of the etiopathogenesis with stress on the recent concepts related to this chronic Indian Disease.
Key Words: Areca nut, Arecoline, Cytokines, HLA typing, Oral precancer, Oral submucous fibrosis. Introduction:
Oral submucous fibrosis (OSF), now globally accepted as an Indian disease, has one of the highest rates of malignant transformation amongst potentially malignant oral lesions and conditions. The condition has also been described as idiopathic scleroderma of mouth, idiopathic palatal fibrosis and sclerosing stomatitis. It was first described three decades ago by Pindborg & Sirsat (1966). The hallmark of the disease is submucosal fibrosis that affects most parts of the oral cavity, pharynx and upper third of the esophagus leading to dysphagia and progressive trismus due to rigid lips and cheeks. The disease is predominantly seen in Asian countries, prevalence being more in India (Fig. 1). Recent epidemiological data indicates that the number of cases of OSF has risen rapidly in India from an estimated 250,000 cases in 1980 to 2 million cases in 1993. The reasons for the rapid increase of the disease are reported to be due to an upsurge in the popularity of commercially prepared areca nut preparations (pan masala) in India(Ranganathan et al, 2004) and an increased uptake of this habit by young people(Gupta et al, 1998) due to easy access, effective price changes and marketing strategies. Oral Submucous fibrosis is diagnosed on the basis of clinical criteria including oral ulceration, paleness of the oral mucosa and burning sensation (particularly in the presence of spicy foods), hardening of the tissue and presence of characteristic fibrous bands. The fibrosis involves the lamina propria and the submucosa and may often extend into the underlying musculature resulting in the deposition of dense fibrous bands giving rise to the limited mouth opening which is a hallmark of this disorder.
Etiology:
Epidemiological data and intervention studies suggest that areca nut is the main aetiological factor for OSF (Pindborg et al, 1984; Seedat & Van Wyk, 1988; Sinor et al, 1990; Maher et al, 1994; Murti et al 1995; Merchant et al, 1997; Shah & Sharma, 1998; Yang et al, 2001; Farrand et al, 2001; IARC, 2004; Jacob et al, 2004). Other etiological factors suggested are chillies, lime, tobacco, nutritional deficiencies such as iron and zinc, immunological disorders, and collagen disorders. Extensive research is being done on the Indian supari ( areca nut / betel nut, Fig. 2).
39 Vol 1 - July 08
Fig. I: Grey areas demonstrate regions which have betel quid chewing habits in south-east Asia with India being the most affected country. -------------------------------------------------------------------------------------Correspondence Author: Dr. Shubhangi Mhaske, Associate Professor & Head, Department of Oral & Maxillofacial Pathology, Peoples Dental Academy, Bhanpur, Bhopal-462010 (M.P.) Ph. 9893778647 E-mail : drmhaske1234@rediffmail.com Peoples Journal of Scientific Research
Oral submucous fibrosis - Current Concepts -------------------- M K Gupta, S Mhaske, R Ragavendra & Imtiyaz.
oral mucosa and causing neurotropic disorder .This view was further supported by the finding that, addition of slaked lime Ca(OH)2 to areca nut in pan facilitates hydrolysis of arecoline to arecaidine (more potent than arecolin) making this agent available in the oral environment. Tobacco & Lime: These are known irritants and causative factors in oral malignancy. They may act as local irritants. The commercially freeze dried products such as Pan masala, Gutka and Mawa (areca, tobacco and lime) have high concentrates of areca nut per chew and appear to cause OSF more rapidly than by self prepared conventional betel quid which contain smaller amounts of areca nut. (Shah & Sharma, 1998; Sinor et al, 1990). Chillies: The use of chillies (Capsicum annum and Capsicum frutescence) has been thought to play an etiological role in oral submucous fibrosis. Capsaicin, which is vanillylamide of 8-methyl-6-nonenic acid, is the active ingredient of chillies, play an etiological role in oral submucous fibrosis (Rajendran, 1994). Nutritional deficiency: A subclinical vitamin B complex deficiency has been suspected in cases of OSF with vesiculations and ulcerations of oral cavity. The deficiency could be precipitated by the effect of defective nutrition due to impaired food intake in advanced cases and may be the effect, rather than the cause of the disease (Rajendran, 1994). Defective iron metabolism: Microcytic hypochromic anemia with high serum iron has been reported in submucous fibrosis (Rajendran, 1994). Collagen disorders: Oral submucous fibrosis is thought to be a localized collagen disease of oral cavity. It is linked to scleroderma, rheumatoid arthritis, Duputreyens contracture and intestinal fibrosis. A link between scleroderma and oral submucous fibrosis has also been suspected on the basis of similarity of histological characteristics (Tsai et al, 1999; Tilakratne et al, 2005).
40 Vol 1 - July 08
Fig. II:Areca nut Indian supari is the main cause for submucous fibrosis
Areca nut: The term areca nut is used to denote the unhusked whole fruit of the areca nut tree and term betel nut is used exclusively to refer to the inner kernel or seed which is obtained after removing husk. The betel nut has psychotropic and anti helminthic property due to presence of areca alkaloids. Four alkaloids have been conclusively identified in biochemical studies, arecoline, arecaidine, guvacine & guvacoline, of which arecoline is the main agent. These alkaloids have powerful parasympathetic properties which produce euphoria and counteract fatigue. Nitrosation of arecoline leads to the formation of a reca nut specific nitrosamine namely nitrosoguvacoline, nitrosoguvacine and 3-methyl nitrosominopropionitrile, which alkylate DNA. Metabolism of these areca nut specific nitrosamine lead to formation of cyanoethyl, which binds with omethyl guanine in DNA. Prolonged exposure to this irritant leads to malignant transformation. Recently suggested pathogenesis of oral submucous fibrosis is by dual action of areca nut. It is suggested that arecoline not only stimulates fibroblastic proliferation and collagen synthesis but also decreases its breakdown. This suggests that arecoline is the active metabolite in fibroblast stimulation. Areca and slaked lime : In a habitual betel nut chewer, oral submucous fibrosis may be caused by the amount of tannic acid contained in the betel nut, the influence of mixed calcium powder and the conditional action of arecoline content in betel nut, affecting the vascular supply of
Peoples Journal of Scientific Research
Oral submucous fibrosis - Current Concepts -------------------- M K Gupta, S Mhaske, R Ragavendra & Imtiyaz.
Immunological disorders: Raised ESR and globulin levels are indicative of immunological disorders. Serum immunoglobulin levels of IgA, IgG and IgM are raised significantly in oral submucous fibrosis. These raised levels suggest an antigenic stimulus in the absence of any infection. Circulating autoantibodies are also present in some cases of oral submucous fibrosis (Canniff et al, 1985).
Pathogenesis:
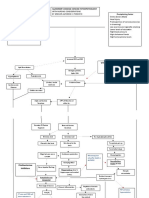
I Collagen accumulation: Oral submucous fibrosis results from increased production of collagen by fibroblasts. In addition to this there is decr eased br eakdown leading to accumulation of excessive amount of collagen. a) Increased Collagen Production: Under the influence of areca nut, fibroblasts differentiated into phenotypes that produce more collagen. The alkaloids present in areca nut, arecadine and arecoline are responsible for this. Arecoline gets converted in to arecadine which is the active metabolite. There is dose dependent increase in production of collagen by fibroblasts under influence of these factors (Flow chart-1;Meghji et al, 1987; Kuo et al, 1995). Various cytokines are increased in oral submucous fibrosis. These are: Transforming growth factor (TGF- ), Platlet derived growth factor (PDFG) and Basic fibroblast growth factor (bFGF). These are fibrogenic growth factors that stimulate collagen production. Another cytokine that has anticollagen effect is Interfran- (IFN- ). This is decreased in OSF. Thus overall there is stimulation of collagen synthesis through different mechanisms (Haque et al, 1998).
b) Stabilization of collagen structure and decreased collagen breakdown: One of the mechanisms that can lead to increased fibrosis is by reduced degradation of collagen by forming a more stable collagen structure. Betel nut contains tannin. Tannin has ability to stabilize collagen by cross-linking it. With the progression of the disease type III collagen is almost completely replaced by type I (Utsunomiya et al, 2005). Type I collagen is more r esistant to degradation than type III. An important finding from these studies is the identification of excess -1 chains relative to -2, suggesting an alteration of collagen molecules during the progression of the disease. Although, the biological function of this trimer is not known, it is regarded as more resistant to degradation than the normal collagen molecule (Tsai et al, 1999). Another component of betel nut that aids this cross-linking is copper. It is a constituent of enzyme lysyl oxidase. This enzyme also causes cross-linking and makes collagen resistant to degradation by collagenase. Due to action of tannin and copper, collagen that is produced in OSF is highly resistant to remodeling and phagocytosis (Tsai et al, 1999). It is fibroblast that brings about remodeling and phagocytosis of collagen. As in OSF, these fibroblasts are affected and phenotypically changed, they cannot degrade collagen. Studies on the effects of arecoline on both normal and OSF fibroblasts in culture revealed an elevated rate of collagen synthesis by OSF fibroblasts as compared to normal fibroblasts.
Flow chart 1: Role of areca alkaloids in OSF (Ghom & Mhaske, 2008). Peoples Journal of Scientific Research 41
Flow chart 2: Role of areca nut in oral submucous fibrosis (Ghom & Mhaske, 2008) Vol 1 - July 08
Oral submucous fibrosis - Current Concepts -------------------- M K Gupta, S Mhaske, R Ragavendra & Imtiyaz.
Although the reason for this elevation is not clear, some authors proposed that it could reflect the clonal selection of a highly fibrogenic cell population in the altered tissue under the influence of local factors such as interleukin-1 from inflammatory cells (Utsunomiya et al, 2005). This leads to decrease in phagocytosis & accumulation of collagen in oral mucosa (Flow chart 2).Glycogen consumption is physiologically related to cellular activity of muscle fibres. Over activity of muscles results in excessive glycogen consumption, leading to its depletion. In areca nut chewers there is overactivity of muscles due to repeated, continuous chewing and use of heavy force to crush the hard nut. This increased muscle activity along with diminished blood supply, following connective tissue changes leads to muscle degeneration and fibrosis.
Higher frequencies of OSF are found in HLA A10, DR3 and DR7 types when compared to an ethnically, regionally and age-matched control group (Cannif et al, 1985; Kuo et al, 1995). Although the data on various HLA types, raised autoantibodies and the detection of immune complexes tend to indicate an autoimmune basis for the disease, substantial number of cases and matched controls may be required to verify these finding (Tilakaratne et al, 2005)
Precancerous nature and malignant transformation:
The precancerous nature of OSF was first described by Paymaster in 1956 when he observed slow growing squamous cell carcinoma (SCC) in one third of the patients with the disease. This was confirmed by various other workers. Pindborg in 1972 put forward five criterias to prove that the disease is precancerous (Pindborg et al, 1984). They included, high occurrence of OSF in oral cancer patients, higher incidence of SCC in patients with OSF, histological diagnosis of cancer without any clinical suspicion in OSF, high frequency of epithelial dysplasia and higher prevalence of leukoplakia among OSF cases. Most of the earlier studies have focused on the prevalence of epithelial dysplasia in OSF. It has so far been the most reliable indicator for predicting potential malignant transformation of an oral precancerous lesion though new markers are emerging (Warnakulasuriya, 2001). Epithelial dysplasia in OSF tissues appeared to vary from 7 to 26% depending on the study population (Pindborg et al, 1967; Murti et al, 1995; Lee et al, 2003). However, according to the current awareness of the disease and some refined criteria for grading dysplasia, it is reasonable to assume that the prevalence of dysplasia is more towards the midway of the reported range. Malignant transformation rate of OSF was found to be in the range of 713%. The hypothesis that dense fibrosis and less vascularity of the corium, in the presence of an altered cytokine activity creates a unique environment for carcinogens from both tobacco and areca nut to act on the epithelium is widely being accepted. It could be assumed that carcinogens from areca nut accumulate over a long period of time either on or immediately below the epithelium allowing the carcinogens to act for a longer duration before it diffuses into deeper tissues.
Vol 1 - July 08
II Increased expression of fibrogenic cytokines:
The most important finding in the various studies was the demonstration of increased expression of fibrogenic cytokines namely TGF -1, PDGF and bFGF in OSF tissues compared to normal (Haque et al, 1998). These observations may suggest that the disease process in OSF may be an altered version of wound healing as recent findings show that the expression of various ECM molecules are similar to those seen in maturation of granulation tissue.(Utsunomiya et al, 2005)
III Genetic polymorphisms predisposing to OSF:
Polymorphisms of the genes coding for TNFhas been reported as a significant risk factor for OSF. TNF - is known to stimulate fibroblastic proliferation in vitro (Vilcek et al, 1986). Evidences suggest that collagen-related genes are altered due to ingredients in the quid.The genes COL1A2, COL3A1, COL6A1, COL6A3 and COL7A1 have been identified as definite TGF- targets and induced in fibroblasts at early stages of the disease. The transcriptional activation of these procollagen genes by TGF- suggests that it may contribute to increased collagen levels in OSF (Rajalalitha & Vali, 2005).
IV OSF as an autoimmune disorder:
OSF is also thought to be an autoimmune disease. The presence of various autoantibodies in varying titers is reported in several studies confirming autoimmune basis to the disease. Few studies have reported on HLA typing of OSF patients and controls.
Peoples Journal of Scientific Research 42
Oral submucous fibrosis - Current Concepts -------------------- M K Gupta, S Mhaske, R Ragavendra & Imtiyaz.
Conclusions:
Various available data suggests that the main causative agents for OSF are the constituents of areca nut, mainly arecoline, whilst tannin may have a synergistic role. Arecoline will interfere with the molecular processes of deposition and/or degradation of extracellular matrix molecules such as collagen. Due to this interference, phagocytic capacity of fibroblast is reduced, because of up or down regulation of key enzymes such as lysyl oxidase and alteration in expression of various ECM molecules. The process may also be influenced by incr eased secretion of inflammatory cytokines, growth factors and decreased production of anti-fibrotic cytokines. Although the above mechanisms may explain the induct ion, maintenance and progression of fibrosis in OSF, further research is required in order to identify the mechanism leading to carcinogenesis in this fibrotic oral mucosa. Nutritional deficiencies may not play a primary role but it could synergize the symptomotology by contributing to epithelial atrophy. Although the involvement of HLA and genetic predisposition has been reported, specific haplotypes have not been determined. The individual mechanisms operating at various stages of the diseaseinitial, intermediate and advancedneed further study in order to propose appropriate therapeutic interventions.
7. Jacob BJ, Straif K, Thomas G, Ramadas K, Mathew B, Zhang ZF, Rangaswamy S, Hashibe M.: Betel quid without tobacco as a risk factor for oral precancers. Oral Oncology, 2004; 40:697704. 8. Kuo MYP, Chen HM, Hahn LJ, Hsieh CC, Chiang CP.: Collagen biosynthesis in human OSF fibroblast cultures. Journal of Dental Research, 1995;74:1783 1788. 9. Lee CH, Ko YC, Huang HL, Chao YY, Sheih TY, Lin LM.: The precancer risk of betel chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in Southern Taiwan. British Journal of Cancer, 2003; 88:366372. 10. Maher R, Lee AJ, Warnakulasuriya KA, Lewis JA, Johnson NW.: Role of areca nut in the causation of oral submucous fibrosis: a case control study in Pakistan. Journal of Oral Pathology & Medicine, 1994;23:6569. 11. Meghji S, Scutt A, Harvey W, Canniff JP.: An in vitro comparison of human fibroblasts from normal and oral submucous fibrosis tissue. Archives of Oral Biology, 1987;32:213215. 12. Merchant AT, Haider SM, Fikree FF.: Increased severity of oral submucous fibrosis in young Pakistani men. British Journal of Oral Maxillofacial Surgery, 1997; 35:284287. 13. Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Metha FS.: Aetiology of oral submucous fibrosis with special reference to the role of areca nut chewing. Journal of Oral Pathology & Medicine, 1995;24:145152. 14. Murti PR, Bhonsle RB, Pindborg JJ, Daftary DK, Gupta PC, Mehta FS.: Malignant transformation rates in oral submucous fibrosis over a 17 year period. Community Dentistry & Oral Epidemiology, 1985;13:340341. 15. Paymaster JC.: Cancer of the buccal mucosa: A clinical study of 650 patients. Cancer, 1956;9:431435. 16. Pindborg JJ, Poulsen E, Zachariah J.: Oral epithelial changes in thirty Indians with oral cancer and oral submucous fibrosis. Cancer, 1967;20:11411146. 17. Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Meth a FS.: Oral submucous fibrosis as a precancerous condition. Scandinavian Journal of Dental Research, 1984;92:224229. 18. Pindborg JJ, Sirsat SM.: Oral submucous fibrosis. Oral Surgery Oral Medicine & Oral Pathology, 1966; 22: 764779. 19. Rajalalitha P, Vali S.: Molecular pathogenesis of oral submucous fibrosiscollagen metabolic disorder. Journal Oral Pathology & Medicine, 2005;34:321 328. 20. Rajendran R.: Oral submucous fibrosis: etiology, pathogenesis and future research. Bulletin of World
43 Vol 1 - July 08
Bibliography:
1. Canniff JP, Batchelor JR, Dodi K, Harvey W.: HLA typing in oral submucous fibrosis. Tissue Antigen, 1985; 26:138142. 2. Farrand P, Rowe RM, Johnston A, Murdoch H.: Prevalence, age of onset and demographic relationships of different Areca nut habits amongst children in Tower Hamlets, London. British Dental Journal, 2001; 190:150154. 3. Ghom A, Mhaske S.: Premalignant lesions and conditions (Chapter 12) In: Textbook of oral pathology. 1st Edn, Jaypee Brothers Medical Publishers (P) Ltd, New Delhi , 2008; pp.194-201 4. Gupta PC, Sinor PN, Bhonsle RB, Pawar VS, Mehta HC. Oral submucous fibrosis in India: a new epidermic. National Medical Journal of India, 1998;11:113116. 5. Haque MF, Harr is M, Meghji S, Barr ett AW.: Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine, 1998;10:713719. 6. International Agency for Research on Cancer (IARC).: Betel-quid and areca nut chewing and some areca nut derived nitrosoamines. Lyon IARC, 2004; 85: 123129.
Peoples Journal of Scientific Research
Oral submucous fibrosis - Current Concepts -------------------- M K Gupta, S Mhaske, R Ragavendra & Imtiyaz.
Health Organisation, 1994;72 (6): 985-996. 22 Ranganathan K, Uma Devi M, Joshua E, Kirankumar K, Saraswathi TR.: Oral submucous fibrosis: a case control study in Chennai South India. Journal Oral Pathology & Medicine, 2004;33:274277. 23. Saeed B, Haque MF, Meghji S, Harris M.: HLA typing in oral submucous fibrosis. Journal of Dental Research, 1997;76:1024. 24 Seedat HA, Van Wyk CW.: Betel nut chewing and oral submucous fibrosis in Durban. South African Medical Journal, 1988;74:572575. 25. Shah N, Sharma PP. Role of chewing and smoking haibts in the aetiology of oral submucous fibrosis (OSF): a case control study. Journal Oral Pathology & Medicine , 1998;27:475479. 26. Sinor PN, Gupta PC, Murti PR, Bhonsle RB, Daftary DK, Mehta FS, Pindborg JJ.: A case-control study of oral submucous fibrosis with special reference to the aetiologic role of areca nut. Journal Oral Pathology & Medicine, 1990;19:9498. 27. Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S.: Oral submucous fibrosis :Review on aetiology and pathogenesis. Oral Oncology, 2005; 42:561-568. 28. Tsai CC, Ma RH, Shieh TY. Deficiency in collagen and fibronectin phagocytosis by human buccal mucosa fibroblasts in vitro as a possible mechanism for oral submucous fibrosis. Journal Oral Pathology & Medicine, 1999;28:5963. 29. Utsunomiya H, Tilakaratne WM, Oshiro K, Maruyama S, Suzuki M, Ida-Yonemochi H, Cheng. J, Sabu T.: Extracellular matrix remodeling in oral submucous fibrosis; its stage-specific modes revealed by immunohistochemistry and in situ hybridization. Journal Oral Pathology & Medicine, 2005;34(8):498507. 30. Vilcek J, Palombella VJ, Henrikson-DeStefano D. Fibroblast growth factor enhancing activity of tumour necrosis factor and its relationship to other polypeptide growth factors. Journal of Experimental Medicine, 1986;163:632643. 31. Warnakulasuriya S.: Histological grading of oral epithelial dysplasia: revisited. Journal of Pathology, 2001;194:294297. 32. Yang YH, Lee HY, Tung S, Shieh TY.: Epidemiological survey of oral submucous fibrosis and leukoplakia in aborigines of Taiwan. Journal Oral Pathology & Medicine, 2001;30:213219.
Peoples Journal of Scientific Research
44
Vol 1 - July 08
You might also like
- RetDocument1 pageRetAsma NawazNo ratings yet
- Dental Specialties RecognizedDocument1 pageDental Specialties RecognizedAsma NawazNo ratings yet
- DENTIST ReferencesDocument3 pagesDENTIST ReferencesAsma NawazNo ratings yet
- 3 2 6Document7 pages3 2 6Asma NawazNo ratings yet
- Oral Submucous FIbrosisDocument13 pagesOral Submucous FIbrosisAsma NawazNo ratings yet
- 3 2 6Document7 pages3 2 6Asma NawazNo ratings yet
- Oral Submucous Fibrosis, Pre MalignantDocument38 pagesOral Submucous Fibrosis, Pre MalignantHaris Mehmood100% (3)
- PJMS MS 145 SPK Vol 9 No 2 2012-8Document7 pagesPJMS MS 145 SPK Vol 9 No 2 2012-8Asma NawazNo ratings yet
- OSF Case Report in Iranian BoyDocument5 pagesOSF Case Report in Iranian BoyAsma NawazNo ratings yet
- Tooth LossDocument3 pagesTooth LossAsma NawazNo ratings yet
- Restorative Management of ToothwearDocument6 pagesRestorative Management of ToothwearSherif ReffatNo ratings yet
- Cervical LesionDocument11 pagesCervical LesionAsma NawazNo ratings yet
- Space LossDocument5 pagesSpace LossAsma NawazNo ratings yet
- Non CariousDocument19 pagesNon CariousAsma NawazNo ratings yet
- Bulimia NervosaDocument4 pagesBulimia NervosaAsma NawazNo ratings yet
- AttritionDocument7 pagesAttritionAsma NawazNo ratings yet
- How To Overcome FailedDocument14 pagesHow To Overcome FailedAsma NawazNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Emcee RubricDocument1 pageEmcee RubricAdi Ruzaini Di EddyNo ratings yet
- Test 1 Điền Lỗ - Anh Ngữ Betty - 2Document8 pagesTest 1 Điền Lỗ - Anh Ngữ Betty - 2Nguyễn Trung HiếuNo ratings yet
- Altered Mental Status: by Diana King, MD, and Jeffrey R. Avner, MDDocument9 pagesAltered Mental Status: by Diana King, MD, and Jeffrey R. Avner, MDchintya claraNo ratings yet
- Application of Leadership TheoriesDocument24 pagesApplication of Leadership TheoriesTine WojiNo ratings yet
- Math 226 Differential Equation: Edgar B. Manubag, Ce, PHDDocument18 pagesMath 226 Differential Equation: Edgar B. Manubag, Ce, PHDJosh T CONLUNo ratings yet
- Coefficient of Friction Guide for Caesar II Pipe Stress AnalysisDocument1 pageCoefficient of Friction Guide for Caesar II Pipe Stress AnalysisAlden Cayaga0% (1)
- SOAL US Adaptif Bhs InggrisDocument15 pagesSOAL US Adaptif Bhs InggrisNur RochiemNo ratings yet
- Multiple Leiomyomas After Laparoscopic Hysterectomy: Report of Two CasesDocument5 pagesMultiple Leiomyomas After Laparoscopic Hysterectomy: Report of Two CasesYosef Dwi Cahyadi SalanNo ratings yet
- Bollinger, Ty M. - Cancer - Step Outside The Box (2009)Document462 pagesBollinger, Ty M. - Cancer - Step Outside The Box (2009)blah80% (5)
- 05 The Milesian NaturalistsDocument18 pages05 The Milesian NaturalistsDr René Mario Micallef, SJ, STDNo ratings yet
- Quiz Template: © WWW - Teachitscience.co - Uk 2017 28644 1Document49 pagesQuiz Template: © WWW - Teachitscience.co - Uk 2017 28644 1Paul MurrayNo ratings yet
- ArgalaDocument4 pagesArgalaTushar Kumar BhowmikNo ratings yet
- Part 3. Scaffolding Children's Understanding and Use of Language PDFDocument7 pagesPart 3. Scaffolding Children's Understanding and Use of Language PDFMaksym BuchekNo ratings yet
- Y3 Module 1 QuizDocument6 pagesY3 Module 1 QuizMohd HattaNo ratings yet
- Ajzen - Constructing A Theory of Planned Behavior QuestionnaireDocument7 pagesAjzen - Constructing A Theory of Planned Behavior QuestionnaireEstudanteSax100% (1)
- Pathophysiology of Alzheimer's Disease With Nursing ConsiderationsDocument10 pagesPathophysiology of Alzheimer's Disease With Nursing ConsiderationsTiger Knee100% (1)
- Lesson Plan For Clothes Fashion TeenagersDocument4 pagesLesson Plan For Clothes Fashion Teenagerschitini170774No ratings yet
- DCF ValuationDocument50 pagesDCF ValuationAnkit JainNo ratings yet
- INFOSEM Final ReportDocument40 pagesINFOSEM Final ReportManasa BanothNo ratings yet
- Seminar Assignments Ticket Vending MachineDocument13 pagesSeminar Assignments Ticket Vending MachineCandy SomarNo ratings yet
- Drama GuidelinesDocument120 pagesDrama GuidelinesAnonymous SmtsMiad100% (3)
- Sea Creatures List, Prices, Shadow Sizes, and Times ACNH - Animal Crossing New Horizons (Switch) Game8Document1 pageSea Creatures List, Prices, Shadow Sizes, and Times ACNH - Animal Crossing New Horizons (Switch) Game8botonlouietNo ratings yet
- Data NormalisationDocument10 pagesData Normalisationkomal komalNo ratings yet
- Employee Engagement Study: Cone CommunicationsDocument16 pagesEmployee Engagement Study: Cone CommunicationsAndrei CristianNo ratings yet
- Analogy and LogicDocument2 pagesAnalogy and LogicCOMELEC CARNo ratings yet
- Islamic Capital Markets: The Role of Sukuk: Executive SummaryDocument4 pagesIslamic Capital Markets: The Role of Sukuk: Executive SummaryiisjafferNo ratings yet
- Interactive Textbook1 1whatis MatterDocument7 pagesInteractive Textbook1 1whatis Matterapi-240094705No ratings yet
- Datos Practicos TIMKENDocument128 pagesDatos Practicos TIMKENneodymioNo ratings yet
- Permutations & Combinations Practice ProblemsDocument8 pagesPermutations & Combinations Practice Problemsvijaya DeokarNo ratings yet
- Aquinas' EthicsDocument33 pagesAquinas' EthicsRBCD INDUSTRIAL SUPPLY100% (1)