Professional Documents
Culture Documents
Morgellons Disease The Mystery Unfolds Virginia R Savely and Raphael B Stricker
Uploaded by
sisterrosettaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Morgellons Disease The Mystery Unfolds Virginia R Savely and Raphael B Stricker
Uploaded by
sisterrosettaCopyright:
Available Formats
"The novel concept of a plant bacterium infecting humans will open a new field of microbiology xenobacteriology.
." Key issues Morgellons disease is a mysterious illness whose cause remains undefined. Patients develop skin lesions with multicolored fluorescent fibers visible under the skin in association with musculoskeletal and neuropsychiatric symptoms. Morgellons disease is often confused with delusional parasitosis, but its occurrence in children and in adults without pre-existing or concurrent psychiatric illness suggests that the disease has a somatic origin. Morgellons disease is often found in patients with Lyme disease and related tick-borne coinfections. Preliminary evidence suggests an association between Morgellons disease and infection with Agrobacterium, a plant bacterium that may be harbored by ticks. Treatment of Morgellons disease is empirical and involves the use of antibacterial, antiparasitic and antifungal medications. +++ Expert Review of Dermatology October 2007, Vol. 2, No. 5, Pages 585-591 Morgellons disease: the mystery unfolds Virginia R Savely and Raphael B Stricker Author for correspondence http://nielsmayer.com/morgellons07.pdf http://ettmuller.wordpress.com/2007/10/27/morgellons-disease-the-mystery-unfolds/ Abstract Morgellons disease is a mysterious skin disorder that was first described over 300 years ago. The disease is characterized by fiber-like strands extruding from the skin in association with dermatologic and neuropsychiatric signs and symptoms. Although Morgellons disease has been confused with delusional parasitosis, the occurrence of the disease in children, the lack of pre-existing psychopathology in most patients and the presence of subcutaneous fibers on skin biopsy indicate that the disease has a somatic origin. The association with Lyme disease and the apparent response to antibiotic therapy supports the concept that Morgellons disease may be triggered by an infectious process. Recent studies suggest that infection with Agrobacterium may play a role in the disease. Further clinical and molecular research is needed to unlock the mystery of Morgellons disease. Morgellons disease is a mysterious illness characterized by nonhealing skin lesions associated with
crawling or stinging sensations under the skin and the presence of fiber-like strands and granule-like objects protruding from the lesions [1]. The disease was first described in French children in 1674 by a British physician, Sir Thomas Browne, as follows [2]: Hairs which have most amused me have not been in the face or head, but on the back, and not in men but children, as I long ago observed in that endemial distemper of little children in Languedock, called the Morgellons, wherein they critically break out with harsh hairs on their backs, which takes off the unquiet symptoms of the disease, and delivers them from coughs and convulsion. In 1682, Dr Michel Ettmullers microscopic drawings of objects associated with what was then believed to be a worm infestation of children (Figure 1) appear similar to microscopic views of fibers from present-day sufferers of this disease [2].
Morgellons disease was rediscovered in 2001 by a Pennsylvania housewife, Mary Leitao, who noted skin lesions with protruding fibers in her 2-year-old son. Unable to find medical help for his condition, she founded the Morgellons Research Foundation [101], which began accepting registrations from people with symptoms of this unrecognized disease in 2002. The original focus of the foundation was on skin symptoms, but it soon became evident that other symptoms within this patient group, such as disabling fatigue, life-altering cognitive decline, musculoskeletal pain and mood disorders were of much greater concern. Morgellons disease was initially considered to be a form of delusional parasitosis (DP) by most dermatologists [311]. However, as the disease has become more widely recognized, significant clinical differences from DP have become apparent (as discussed later) [12,13,102]. The recent discovery of a putative infectious cause of the disease supports a somatic etiology of this bizarre skin condition. Case definition The Morgellons Research Foundation lists seven primary signs and symptoms of the condition: Skin lesions accompanied by intense itching Crawling sensations on and under the skin, often compared to insects moving, stinging or biting (cutaneous dysesthesia) Fibers, which can be white, blue, red or black, in and on the lesions Fatigue significant enough to interfere with daily activity Musculoskeletal pain Inability to concentrate and difficulty with short-term memory Behavioral changes
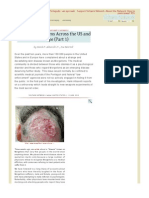
Patients with Morgellons disease typically have skin lesions that can range from minor to disfiguring in appearance (Figures 2 & 3). A network of blue, red, white and black fibers under the skin of these patients as well as blue, black and white fibers protruding from the lesions can be visualized using a 30 hand-held digital microscope (Figure 3). Attempts to remove the tough filaments protruding from skin lesions induces shooting pains radiating from the site, indicating that the filaments are not simply adsorbed to the skin and may be quite resistant to extraction.
The fiber-like material can be observed in skin lesions as either single strands or what appear to be balls of fibrous material that may demonstrate autofluorescence (Figure 4) [1]. Patients frequently describe this material as fibers, fiber balls or fuzz balls. Granules protruding from the skin of patients can often be seen microscopically to have one or more fibers attached at the ends. Patients often describe these granules as seeds, eggs or sand [1]. Many individuals report material described as black specks or black oil. Some patients have no observable skin lesions, with the skin sensations and fibrous, granular or black material being the only visible indicator of the disease [1]. According to statistics from the Morgellons Research Foundation, 95% of patients report symptoms of disabling fatigue, self-described brain fog or attention deficit, while 50% of patients report problems with fibromyalgia, joint pain and sleep disorders. Other symptoms include hair loss, decline in vision, neurological disorders and occasionally tooth decay despite the lack of caries or gingivitis. Most patients are unable to continue working, and those who do work report that they do not function optimally [1].
Patients with this disease are often diagnosed with a psychosomatic illness by the dermatologists whom they consult for care [311]. Typically, patients have sought help from 1040 physicians who often make a diagnosis of DP without a thorough examination and interpret the obvious open sores on the patients skin as attempts at self-mutilation [12]. One patient described his experience with Morgellons disease in this way [1]: I have had this disease for 20 years. I spent the first 10 years going from doctor to doctor for help. I spent the last 10 years just living with it, knowing that no one would ever help me. Patients often exhibit psychopathology that appears to be directly attributable to the disease. This fact confounds the clinical picture for patients as they seek validation for an insidious dermatologic condition while exhibiting obvious symptoms of mental illness [1]. It appears that the putative underlying infectious disease, left unrecognized and untreated, can cause psychopathology in many patients. This aspect of Morgellons disease appears to be analogous to the clinical course of infection with Treponema pallidum, the spirochetal agent of syphilis that can cause dermatologic and neuropsychiatric pathology at different stages of infection. The syphilis model underscores a basic premise of psychosomatic dermatology: the diagnosis of a delusional disorder must be based on the presence of a primary psychiatric disorder rather than the absence of known dermatopathology [1]. In the case of Morgellons disease, a poorly characterized dermatologic condition appears to trigger secondary psychopathology that may be wrongly confused with a primary delusional disorder [1]. Epidemiology & transmission The states of California, Texas and Florida in the USA appear to have the highest number of reports of this disease, with primary clusters noted in Los Angeles and San Francisco (CA) and Houston, Dallas and Austin (TX). California accounts for 26% of cases in the USA. All 50 states and 15 nations, including Canada, England, Australia and The Netherlands have reported cases of Morgellons disease. The total number of registrations on the Morgellons Research Foundation website is presently over 10,000, which is believed to be a fraction of the actual number of cases. We currently follow more than 200 Morgellons patients in our practice in San Francisco. Initially, Morgellons disease was thought to be more prevalent in nurses and teachers, but that finding has not been substantiated. The male to female ratio is approximately 1:1 according to the Morgellons
Research Foundation. The disease affects all age groups including children, but the prevalence in children is unknown at present. There is often a history of traumatic exposure to plants, dirt or soil, such as gardening, landscaping, farming, camping or other outdoor activities. The association with plant exposure has implications for the etiology of the disease (as discussed later). It appears that skin lesions and fibers may not be present in all individuals with this disease, since family members of patients often report similar systemic symptoms without skin lesions [1]. Whether the disease is transmissible by human contact remains unclear, but preliminary evidence suggests that it is not. Although most sufferers are fearful of infecting family members, the rare instances where all family members are affected appear to have a common exposure to an inciting agent [1]. Patients have reported symptoms of this disease in their pets [1]. The majority of reports involve dogs, but cats appear to be increasingly affected. Skin lesions fitting the description of Morgellons disease have also been reported in horses, and horse owners have observed fibers associated with skin lesions on their animals by using a lighted 30 hand-held microscope [1]. Pathophysiology Skin biopsies of patients typically reveal nonspecific pathology, or an inflammatory process with no observable pathogens [1]. Several biopsies have shown fibrous material projecting from inflamed epidermal tissue. Often the biopsies are reported to contain textile fibers located in the dermal region rather than being adherent to the skin. How these fibers arrive at a subcutaneous location remains unexplained. It has been postulated that the fibrous (and other) material associated with skin lesions may be linked to the biofilm of a bacteria, Stenotrophomonas maltophila [Schwartz G, Pers. Comm. 2005], but this hypothesis has not been confirmed [13]. Recent studies indicate that Morgellons fibers are resistant to chemical solubilization and heating, making analysis difficult by conventional means [13]. There is preliminary information that some Morgellons fibers are made of cellulose, but this information has neither been formally evaluated nor confirmed [1]. In a classic study, Hall et al. identified fibers composed of a celluloseprotein complex as a minor constituent of mammalian connective tissue, and increased amounts of these fibers were noted in tissue from patients with scleroderma and other pathological skin conditions [14]. More recent research has demonstrated synthesis of cellulose fibers by certain Gram-negative bacteria and fungi [1518]. Studies using newer carbohydrate microarray technologies may unlock the mystery of these fibers [19]. By contrast to mammalian studies, cellulose is known to be produced in copious quantities by plant bacteria [15]. This observation led to investigation of plant pathogens in Morgellons disease. In a preliminary study, skin biopsies from Morgellons patients revealed evidence of infection with Agrobacterium, which causes crown gall disease in plants [20]. Agrobacterium is unique among plant pathogens because it can infect human cells in vitro, and sporadic cases of catheter sepsis due to Agrobacterium have been reported in immunocompromised patients [21,22]. The presence of Agrobacterium in Morgellons skin lesions suggests that this plant bacterium may be involved in the pathogenesis of the disease. Further investigation is required to confirm this novel hypothesis. Differential diagnosis & association with Lyme disease The differential diagnosis of Morgellons disease includes DP, drug-induced formication, scabies, the tropical dermatoses and the perforating dermatoses. DP, or Eckboms syndrome, is a psychiatric disorder in which patients mistakenly believe that they are infested with a skin parasite [311]. Patients often refuse to accept a psychiatric diagnosis for their skin
symptoms and continue to insist that they have a parasitic infestation. Physicians are taught about the matchbox sign of DP, so-called because patients use a matchbox to carry samples of hair, lint or fuzz to the physician in an attempt to provide evidence of the agent responsible for their torment. When two people describe symptoms of DP, the condition is termed folie deux (madness of two) [7]. There is also folie trois (madness of three) and folie quatre (madness of four). DP affecting all members of a family is considered folie famille (madness of family) [8]. Antipsychotic medications such as pimozide or risperidone are often prescribed for these patients [3,8]. A recent study from India found that DP occurred in only 2% of patients with primary psychiatric conditions (bipolar disorder, depression, schizophrenia or anxiety disorder). Conversely, 100% of patients diagnosed with DP had an underlying primary psychiatric diagnosis [23]. Morgellons disease is distinct from DP in a number of ways. First, the disease may be diagnosed in children who generally do not develop delusional disorders. Second, while DP almost always occurs in patients with underlying psychopathology who otherwise can function fairly normally [24], most Morgellons patients have no signs of pre-existing psychiatric illness and have significant impairment in functioning. Third, the epidemiology of Morgellons disease (outlined previously) differs from delusional disease: the latter is extremely rare in the USA with a prevalence estimated at 0.03%, and usually occurs in middle-aged or older women. Finally, the association with a putative bacterial agent suggests that Morgellons disease has a somatic rather than a psychiatric etiology [2427]. Drug-induced formication (a false sensation of bugs crawling on the skin) is a sensory hallucination induced by psychostimulant drugs such as cocaine or amphetamines [28]. The hallucination has been referred to as coke mites, meth mites or amphetamites depending on the offending agent. Patients with this drug-induced disorder often develop skin lesions due to picking and scratching of accessible skin areas. By contrast, patients with Morgellons disease usually have no history of psychostimulant drug use, and their skin lesions feature perforating fibers that occur at inaccessible locations on the body. Scabies is a pruritic skin disease caused by infestation with the microscopic mite Sarcoptes scabei [29]. The infestation is found primarily in the webbing between the fingers and in the skin folds on the wrist, elbow or knee. Intense itching may occur especially at night and over most of the body. Lack of the typical scabies skin distribution and the absence of mite infestation distinguishes scabies from Morgellons disease. The tropical dermatoses are pruritic skin disorders caused by parasitic infections, including filariasis, onchocerciasis and cutaneous larva migrans [3032]. These dermatoses are prevalent in the tropics, and the skin lesions are associated with eosinophilia, lymphatic obstruction (filariasis), subcutaneous nodules (onchocerciasis) or serpiginous skin lesions (larva migrans). The causative nematode is detected in skin biopsy samples. By contrast, tropical travel and eosinophilia are not commonly reported in Morgellons patients and nematodes have not been identified in skin biopsy samples from these individuals. However, the tropical dermatoses may respond to treatment with antiparasitic medications such as ivermectin and thiabendazole [3032] and antiparasitic therapy may be useful in treating Morgellons disease. The perforating dermatoses are a group of skin diseases characterized by elimination of elastic or collagen fibers from the upper dermis through the skin [3336]. There are five members of this group: Kyrles disease, perforating folliculitis, reactive perforating collagenosis, elastosis perforans serpiginosa and acquired perforating dermatosis [33]. The perforating dermatoses are associated with hereditary diseases including EhlersDanlos syndrome, osteogenesis imperfecta, Down syndrome and
Wilsons disease. They may also be seen in patients with brittle diabetes mellitus or dialysis-dependent renal failure [3336]. These dermatoses are distinct from Morgellons disease because the protruding fibers are made up of elastic or collagenous tissue that is easily identified by histopathology [34]. In addition, Morgellons patients generally do not have the rare hereditary conditions or severe metabolic abnormalities associated with the perforating dermatoses. Conversely, systemic infection and neuropsychiatric symptoms have not been associated with these dermatoses, and they do not respond to antibiotic therapy [3336]. Many patients with Morgellons disease have positive testing for tick-borne pathogens including Borrelia burgdorferi the spirochetal agent of Lyme disease [1]. It appears that there may be a connection between the two diseases, with one infection possibly predisposing the individual to a second infectious agent. Whether all patients with Morgellons disease also have Lyme borreliosis remains to be determined. An intriguing possibility is that the agent of Morgellons disease is transmitted by a tick bite. Since ticks are known to harbor plant bacteria [37], transmission of these pathogens via tick bite may allow the otherwise nonpathogenic plant bacteria to induce disease in the human host. Support for this hypothesis is lacking at present. Treatment Treatment of Morgellons disease is problematic. Since the etiologic agent of the disease is unknown at present, the optimal treatment approach remains undefined [1]. Antipsychotic medications used to treat DP have not been effective for Morgellons patients, although compliance with these medications has been questionable in many cases. Treatment with antibiotics appears to be the most effective therapy for the disease [1,12]. Although standard antibiotic therapy for Lyme disease may be helpful in some cases, the response rate appears to be variable. Treatment with antiparasitic medications may be effective in other cases, and combinations of antibacterial, antiparasitic and antifungal medications may be useful. It is noteworthy that Agrobacterium is extremely resistant to antibiotics in immunocompromised patients, even though the microbe appears to be sensitive to these antimicrobials in vitro [21]. A similar situation may exist in Morgellons patients who harbor an adapted strain of the bacteria. This possibility and its ramifications for the treatment of Morgellons disease require further study. Discussion Morgellons disease remains controversial, and many physicians are convinced that the disease is simply a manifestation of DP [911]. In this regard, it is noteworthy that the medical world has always been reluctant to adopt new paradigms or concepts of disease [38]. As examples, the Hungarian physician Ignaz Semmelweis was ridiculed in the 1850s for suggesting that childbed fever was caused by an infectious agent, and the term Semmelweis reflex was coined to describe automatic dismissal or rejection of scientific information without thought, inspection, or experiment [39]. In the early 1900s, syphilitic patients were confined to mental institutions before it was realized that they were suffering from an infectious disease, while more recently, the medical community took years to accept the fact that peptic ulcer disease is caused by the infectious agent Helicobacter pylori, or that spongiform encephalopathy is caused by a novel prion agent. In the case of Morgellons disease, the medical community may be ignoring an unusual and previously unrecognized infection, consigning patients to frustration and suffering by not validating or attempting to treat this infection [1]. The growing number of patients with symptoms of Morgellons disease who lack the typical features of DP supports the idea that these patients are suffering from a puzzling infectious disease that may cause horrific symptoms and psychiatric sequelae.
Until a formal study of Morgellons disease is accomplished, the cause, transmission and treatment of the disease will remain uncertain [12,13,102]. Following the death of a young Morgellons patient in 2006 and the widespread publicity generated by that incident, the CDC initiated an investigation in an attempt to validate the existence of the disease [40]. The formal report of the CDC investigation is eagerly awaited. Conclusions More than 300 years after its initial description, Morgellons disease remains a medical mystery. In view of the uncertainty surrounding the disease, medical practitioners must look beyond what they have been taught when confronted with new and puzzling symptoms in patients. Rather than being quick to pigeonhole Morgellons patients into a psychiatric diagnosis such as DP, practitioners should take patient complaints seriously and keep an open mind about this potentially novel infectious disease. Recognition of Morgellons disease serves as a reminder to medical practitioners that we have much to learn from listening to the patient. At the same time, newer molecular techniques will hopefully unlock the secrets of this devastating illness. Expert commentary Owing to uncertainty about the cause of Morgellons disease, the optimal treatment for this dermatologic syndrome remains unclear. At this time, empirical therapy with combinations of antibacterial, antiparasitic and antifungal medication is indicated to control the disease. Treatment of associated Lyme disease and related tick-borne coinfections is also essential in these patients. Once the causative agent of Morgellons disease is defined, more effective treatment strategies can be developed. Five-year view As more patients are diagnosed with Morgellons disease, the syndrome will be officially recognized by government agencies as an emerging infectious disease. Molecular research will confirm that the disease is caused by infection with a plant bacterium that is transmitted by a tick bite. Further work with this bacterial agent will define the optimal antibiotic therapy for patients with the disease. Development of more potent disease-specific antibiotics will allow more effective therapy of Morgellons patients. The novel concept of a plant bacterium infecting humans will open a new field of microbiology xenobacteriology. Key issues Morgellons disease is a mysterious illness whose cause remains undefined. Patients develop skin lesions with multicolored fluorescent fibers visible under the skin in association with musculoskeletal and neuropsychiatric symptoms. Morgellons disease is often confused with delusional parasitosis, but its occurrence in children and in adults without pre-existing or concurrent psychiatric illness suggests that the disease has a somatic origin. Morgellons disease is often found in patients with Lyme disease and related tick-borne coinfections. Preliminary evidence suggests an association between Morgellons disease and infection with Agrobacterium, a plant bacterium that may be harbored by ticks. Treatment of Morgellons disease is empirical and involves the use of antibacterial, antiparasitic and antifungal medications. Acknowledgments The authors thank Vitaly Citovsky, Kerry Clark, Keith Clay, William Harvey, Alan MacDonald, James Schaller, Randy Wymore and Adi Zaltsman for helpful discussion. We also thank Mary Leitao, Pat Smith and Cindy Casey for their invaluable contributions, and Mr and Mrs Robert Weinke for their
generous support. This article is dedicated to the memory of Charles Holman. Financial & competing interests disclosure RB Stricker serves on the advisory panel for QMedRx Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript. References Papers of special note have been highlighted as: of interest of considerable interest 1 Savely VR, Leitao MM, Stricker RB. The mystery of Morgellons disease: infection or delusion? Am. J. Clin. Dermatol. 7, 15 (2006). First description of Morgellons disease in peer-reviewed medical literature. [CrossRef][CrossRef] [Medline][Medline] 2 Kellett CE. Sir Thomas Browne and the disease called the Morgellons. Ann. Medical History 7, 467 469 (1935). Historical view of Morgellons disease. 3 Koo J, Gambla C. Delusions of parasitosis and other forms of monosymptomatic hypochondriacal psychosis. General discussion and case illustrations. Dermatol. Clin. 14, 429438 (1996). [CrossRef] [CrossRef] [Medline][Medline] 4 Koo J, Lee CS. Delusions of parasitosis. A dermatologists guide to diagnosis and treatment. Am. J. Clin. Dermatol. 2, 285290 (2001). Review of delusional parasitosis. [CrossRef][CrossRef] [Medline] [Medline] 5 Wilson FC, Uslan DZ. Delusional parasitosis. Mayo Clin. Proc. 79, 1470 (2004). [Medline][Medline] 6 Bourgeois ML, Duhamel P, Verdoux H. Delusional parasitosis: folie a deux and attempted murder of a family doctor. Br. J. Psychiatry 161, 709711 (1992). [Medline][Medline] 7 Daniel E, Srinivasan TN. Folie a famille: delusional parasitosis affecting all the members of a family. Indian J. Dermatol. Venereol. Leprol. 70, 296297 (2004). [Medline][Medline] 8 Lorenzo CR, Koo J. Pimozide in dermatologic practice: a comprehensive review. Am. J. Clin. Dermatol. 5, 339349 (2004). [CrossRef][CrossRef] [Medline][Medline] 9 Murase JE, Wu JJ, Koo J. Morgellons disease: a rapport-enhancing term for delusions of parasitosis. J. Am. Acad. Dermatol. 55, 913914 (2006). [CrossRef][CrossRef] [Medline][Medline] 10 Waddell AG, Burke WA. Morgellons disease? J. Am. Acad. Dermatol. 55, 914915 (2006). [CrossRef][CrossRef] [Medline][Medline] 11 Koblenzer CS. The challenge of Morgellons disease. J. Am. Acad. Dermatol. 55, 920922 (2006). [CrossRef][CrossRef] [Medline][Medline] 12 Harvey WT. Morgellons disease. J. Am. Acad. Dermatol. 56, 705706 (2007). [CrossRef][CrossRef] [Medline][Medline] 13 Wymore RS, Casey RL, Allen RW et al. Physical evidence in Morgellons disease. Presented at: The 14th International Molecular Medicine Tri-Conference. San Francisco, CA, USA, 27 February2 March 2007. 14 Hall DA, Happey F, Lloyd PF, Saxl H. Oriented cellulose as a component of mammalian tissue. Proc. R. Soc. Lond. B Biol. Sci. 151, 497516 (1960). [Medline][Medline] 15 Matthysse AG, White S, Lightfoot R. Genes required for cellulose synthesis in Agrobacterium tumefaciens. J. Bacteriol. 177, 10691075 (1995). [Medline][Medline] 16 Bertocchi C, Delneri D, Signore S, Weng Z, Bruschi CV. Characterization of microbial cellulose from a high-producing mutagenized Acetobacter pasteurianus strain. Biochim. Biophys. Acta 1336, 211217 (1997). [Medline][Medline] 17 Brown RM. Cellulose structure and biosynthesis. Pure Appl. Chem. 71, 204212 (1999). 18 Ude S, Arnold DL, Moon CD, Timms-Wilson T, Spiers AJ. Biofilm formation and cellulose
expression among diverse environmental Pseudomonas isolates. Environ. Microbiol. 8, 19972011 (2006). [CrossRef][CrossRef] [Medline][Medline] 19 Wang D. Carbohydrate microarrays. Proteomics 3, 21672175 (2003). [CrossRef][CrossRef] [Medline][Medline] 20 Stricker RB, Savely VR, Zaltsman A, Citovsky V. Contribution of Agrobacterium to Morgellons disease. J. Invest. Med. 55, S123 (2007). First description of Agrobacterium in Morgellons patients. 21 Giammanco GM, Pignato S, Santangelo C, Grimont PA, Grimont F, Giammanco G. Molecular typing of Agrobacterium species isolates from catheter-related bloodstream infections. Infect. Control Hosp. Epidemiol. 25, 885887 (2004). Overview of opportunistic Agrobacterium bacteremia in humans. [CrossRef][CrossRef] [Medline][Medline] 22 Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl Acad. Sci. USA 98, 18711876 (2001). Seminal article showing transformation of human cells by Agrobacterium. [CrossRef][CrossRef] [Medline][Medline] 23 Kuruvila M, Gahalaut P, Zacharia A. A study of skin disorders in patients with primary psychiatric conditions. Indian J. Dermatol. Venereol. Leprol. 70, 292295 (2004). [Medline][Medline] 24 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Text Revision (DSM-IV-TR). APA, Washington, DC, USA (2000). 25 Manschreck TC. Delusional disorder: the recognition and management of paranoia. J. Clin. Psychiatry 57(Suppl. 3), 3238 (1996). Overview of epidemiology of delusional disorders. [Medline] [Medline] 26 Koo J, Gambla C, Fried R. Pseudopsychodermatologic disease. Dermatol. Clin. 14, 525530 (1996). Reminder that somatic diseases may be misdiagnosed as psychosomatic disorders. [CrossRef] [CrossRef] [Medline][Medline] 27 Ford EB, Calfee DP, Pearson RD. Delusions of intestinal parasitosis. South. Med. J. 94, 545547 (2001). [Medline][Medline] 28 Clark T. Photoessay: formication. Consultant 46, 15121513 (2006). 29 Wendel K, Rompalo A. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin. Infect. Dis. 35(Suppl. 2), S146S151 (2002). [CrossRef][CrossRef] [Medline][Medline] 30 Melrose WD. Chemotherapy for lymphatic filariasis: progress but not perfection. Expert Rev. Anti Infect. Ther. 1, 571577 (2003). [Abstract] 31 Brieger WR, Awedoba AK, Eneanya CI et al. The effects of ivermectin on onchocercal skin disease and severe itching: results of a multicentre trial. Trop. Med. Int. Health 3, 951961 (1998). [CrossRef] [CrossRef] [Medline][Medline] 32 Shinkar RM, Stocks R, Thomas E. Cutaneous larva migrans, creeping eruption, sand worm. Arch. Dis. Child. 90, 998 (2005). [Medline][Medline] 33 Sehgal VN, Jain S, Thappa DM, Bhattacharya SN, Logani K. Perforating dermatoses: a review and report of four cases. J. Dermatol. 20, 329340 (1993). [Medline][Medline] 34 Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. Evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch. Dermatol. 125, 10741078 (1989). [CrossRef][CrossRef] [Medline][Medline] 35 Mehta RK, Burrows NP, Payne CM, Mendelsohn SS, Pope FM, Rytina E. Elastosis perforans serpiginosa and associated disorders. Clin. Exp. Dermatol. 26, 521524 (2001). [CrossRef][CrossRef] [Medline][Medline] 36 Becuwe C, Dalle S, Ronger-Savle S et al. Elastosis perforans serpiginosa associated with pseudopseudoxanthoma elasticum during treatment of Wilsons disease with penicillamine. Dermatology 210, 6063 (2005). [CrossRef][CrossRef] [Medline][Medline] 37 Benson MJ, Gawronski JD, Eveleigh DE, Benson DR. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 70(1), 616620 (2004). Demonstration of plant bacteria in ticks.
[CrossRef][CrossRef] [Medline][Medline] 38 Kuhn TS. The Structure of Scientific Revolutions (2nd Edition). University of Chicago Press, IL, USA (1970). 39 Grant GJ, Grant AH, Lockwood CJ. Simpson, Semmelweis, and transformational change. Obstet. Gynecol. 106, 384387 (2005). [Medline][Medline] 40 Morris E. Mysterious Morgellons disease prompts US investigation. Nature Med. 12, 982 (2006). Websites 101 Morgellons Research Foundation www.morgellons.org/ 102 Morgellons disease: managing a mysterious skin condition. www.mayoclinic.com/health/morgellons-disease/SN00043 Affiliations Virginia R Savely International Lyme and Associated Diseases Society (ILADS), PO Box 341461, Bethesda, MD 20827-1461, USA. lymesf@gmail.com; www.ilads.org Raphael B Stricker International Lyme and Associated Diseases Society (ILADS), PO Box 341461, Bethesda, MD 20827-1461, USA. rstricker@usmamed.com; http://www.ilads.org +++ Burisch Crain Burisch Crain Stricker Monsanto GM GMO Morgellons we think that this plant bacteria may be involved in the cause of the disease agrobacterium We know that ticks can carry something like 40 different bacteria and so it is possible the ticks are picking up the plant bacteria and transmitting it along with the Lyme Disease infecting people through their bite, said Dr. Stricker. +++ Agrobacterium and Morgellons Disease, A GM Connection? http://www.morgellons-research.org/morgellons/agrobacteriumAndMorgellonsFull.pdf Excerpt The Agrobacterium vector system for gene transfer
Since the discovery in the 1970s that Agrobacterium can transfer genes into plants causing crown gall disease, the soil bacterium has been developed into a vector for inserting desirable genes into the plant genome to create transgenic (GM) plants [12].
Agrobacterium transfers T-DNA a small region of approximately 5 to 10 percent of a resident tumour-inducing (Ti) or root-inducing (Ri) plasmid into numerous species of plants; and as later turns
out, also to fungi, algae, and even animal and human cells [13, 14] (see main text). Transfer requires three major elements [13]: T-DNA border direct repeat sequences of 25 base pairs that flank the T-DNA and delineate the region transferred into the host, the virulence (vir) genes located on the Ti/Ri plasmid, and various genes on the bacterial chromosome. Plant genes are also involved in the successful integration of T-DNA [15]. The T-DNA contains oncogenes (cancer genes or gene for forming tumours) and genes for synthesizing opines; none of which is essential for T-DNA transfer, so they can be deleted and replaced with genes of interest and selectable markers. Furthermore, the vir genes and T-DNA region need not be on the same replicating plasmid. This gave rise to the binary vector systems in which T-DNA and the vir genes are located on separate replicating units. The T-DNA containing unit is the binary vector and contains also the origin(s) of replication that could function both in E. coli and Agrobacterium tumefaciens, and antibiotic resistance marker genes used to select for the presence of the binary vector in bacteria. The replicating unit containing the vir genes is the helper plasmid. Strains of Agrobacterium harbouring the two separate units are considered disarmed if they do not contain oncogenes that could be transferred to a plant. The association of Morgellons Disease with dirt and soil where Agrobacterium lives, the widespread use of Agrobacterium in genetic engineering of plants, and the ability of Agrobacterium to infect human cells, all point towards a possible role of genetic engineering in the aetiology of Morgellans disease via Agrobacterium. Extensive genetic manipulation of Agrobacterium does have the potential to transform it into an aggressive human pathogen. Genetic engineering is nothing if not enhanced and facilitated horizontal gene transfer and recombination, which is widely acknowledged to be the main route for creating new pathogens. MaeWan Ho was among an international panel of scientists have raised this very issue in 1998, calling for a public enquiry into the possible contributions of genetic engineering biotechnology to the
aetiology of infectious diseases which has greatly increased since genetic engineering began in the 1970s [16]. The epidemiological data of Morgellons Disease are very incomplete, and the Morgellons Research Foundations registry of more than 12 000 families afflicted worldwide is almost certainly only a fraction of the emerging epidemic. Still, it is significant that the majority of the cases are in the United States, the first country to release GM crops and remaining the top producer ever since. There are other findings implicating Agrobacterium in transgenic plants released into the environment, particularly during the early years of field trials, when knowledge was poor and safety measures not as stringent as they may be today. [Me: If I am reading this correctly, then Morgellons sufferers are just unlucky enough to have been bitten by a tick or other insect or arachnid that just happens to be harboring the plant bacteria Agrobacterium. Many reasons to avoid Monsanto GM GMO genetically-modified organisms in our diet, but especially bad for those of us infected from the bite of a tick, which itself has been infected by plant bacteria plant bacteria introduced into our soil by Monsanto, Corporate America, their allies in Congress and a complicit media damned by their sickening silence. I can afford to travel to San Francisco and pay for treatment out of pocket. But many can't and they suffer needlessly owing to the callousness of the government of the United States of America. I pray a philanthropist will start a foundation dedicated to covering the travel and medical expenses of these unfortunates until our once great nation is good, again. Rose]
You might also like
- Morgellons Disease, Illuminating An Undefined Illness: A Case SeriesDocument8 pagesMorgellons Disease, Illuminating An Undefined Illness: A Case SeriesChristine HeidtNo ratings yet
- Military Industrial Complex Medical Industrial Complex: Bio-Techno-Terrorist EaraFrom EverandMilitary Industrial Complex Medical Industrial Complex: Bio-Techno-Terrorist EaraNo ratings yet
- Agrobacterium MorgellonsDocument9 pagesAgrobacterium MorgellonsRui Baptista PereiraNo ratings yet
- MORGELLONS DISEASE Kharkov National Medical University, UkraineDocument8 pagesMORGELLONS DISEASE Kharkov National Medical University, UkrainethisdraftNo ratings yet
- MorgellonsDocument3 pagesMorgellonsUul Parello OllaolloNo ratings yet
- Oklahoma State University Findings On MorgellonsDocument4 pagesOklahoma State University Findings On MorgellonsSpace_Hulker100% (5)
- Insect PoliticsDocument5 pagesInsect PoliticsFOBCNo ratings yet
- Morgellons ObservationsDocument9 pagesMorgellons ObservationsThorsteinn ThorsteinssonNo ratings yet
- Morgellons Morphology Confirmed.Document3 pagesMorgellons Morphology Confirmed.Thorsteinn ThorsteinssonNo ratings yet
- GMO and Morgellon SDocument4 pagesGMO and Morgellon SCliffordNo ratings yet
- In Their Own Words: Symptoms of Morgellons and Neuro-Cutaneous Syndrome (NCS)Document8 pagesIn Their Own Words: Symptoms of Morgellons and Neuro-Cutaneous Syndrome (NCS)domasjeffersonNo ratings yet
- Morgellons Victims Across The USDocument11 pagesMorgellons Victims Across The USchromelung100% (1)
- Evilfaces Morgellons PDFDocument44 pagesEvilfaces Morgellons PDFAndréNo ratings yet
- Morgellons CanariesDocument7 pagesMorgellons CanariesThorsteinn ThorsteinssonNo ratings yet
- Morgellons Disease, Donna's Testimony About The Miracle Healing She ReceivedDocument5 pagesMorgellons Disease, Donna's Testimony About The Miracle Healing She ReceivedMarkLBNo ratings yet
- New Photos of Morgellons SpecimensDocument22 pagesNew Photos of Morgellons SpecimensThorsteinn Thorsteinsson100% (4)
- ARTICLE - Morgellons and Flying InsectsDocument33 pagesARTICLE - Morgellons and Flying InsectsDaz93% (14)
- Morgellons A New ClassificationDocument6 pagesMorgellons A New Classificationragod2No ratings yet
- Ti Electromagnetic Radiation and Its Effect On The Brain An Insider Speaks Out PDFDocument22 pagesTi Electromagnetic Radiation and Its Effect On The Brain An Insider Speaks Out PDFBozo ZarubicaNo ratings yet
- Morgellons Dr. WymoreDocument2 pagesMorgellons Dr. WymoreSpace_Hulker100% (2)
- Strahlenfolter - International Center Against Abuse of Covert Technologies - Jesse Beltran & Lars Drudgaard Interview On Covert Technologies Affecting Us All - ThetruthdeniedDocument8 pagesStrahlenfolter - International Center Against Abuse of Covert Technologies - Jesse Beltran & Lars Drudgaard Interview On Covert Technologies Affecting Us All - ThetruthdeniedTimo_KaehlkeNo ratings yet
- Inhibition of Growth of the So-called Morgellons Condition Ascorbic Acid Vitamin C, N-Acetyl Cysteine NAC and Glutathione a Sub-micron Cross-domain Bacteria CDB Extremely Resistant to Extinction Morgellons - A Working HypothesisDocument24 pagesInhibition of Growth of the So-called Morgellons Condition Ascorbic Acid Vitamin C, N-Acetyl Cysteine NAC and Glutathione a Sub-micron Cross-domain Bacteria CDB Extremely Resistant to Extinction Morgellons - A Working Hypothesissisterrosetta100% (2)
- Carnicom Institute Research-2003Document173 pagesCarnicom Institute Research-2003Anonymous i71HvPXNo ratings yet
- MorgellonsDocument3 pagesMorgellonsEdward McSweegan, PhD0% (2)
- GMO and Morgellon's DiseaseDocument3 pagesGMO and Morgellon's DiseaseThorsteinn ThorsteinssonNo ratings yet
- U. S. A. Rep. Jim Guest LetterDocument2 pagesU. S. A. Rep. Jim Guest LetterHRC100% (3)
- Nanobots Smart Dust 5G Smart CitiesDocument2 pagesNanobots Smart Dust 5G Smart Citiesjbotha01No ratings yet
- Targeted Individuals (TIs) and Electronic HarassmentDocument4 pagesTargeted Individuals (TIs) and Electronic HarassmentIJRASETPublicationsNo ratings yet
- Strahlenfolter Stalking - TI - Freedom From Covert Harassment and SurveillanceDocument13 pagesStrahlenfolter Stalking - TI - Freedom From Covert Harassment and SurveillanceDieter-Klaus-Klein100% (1)
- Smart Dust and Its Applications: by N.P.Dharani Roll No: 09C31D0617Document33 pagesSmart Dust and Its Applications: by N.P.Dharani Roll No: 09C31D0617vasantha_btechNo ratings yet
- ECFS Filing Detail - 5G, Mind Control, & Electromagnetic WarfareDocument9 pagesECFS Filing Detail - 5G, Mind Control, & Electromagnetic WarfareMayowa Israel AmooNo ratings yet
- Morgellons Exposed - Jan Smith Home PageDocument22 pagesMorgellons Exposed - Jan Smith Home Pagedxguy7100% (6)
- Selected ES and EHS Studies-2018 by ES-UKDocument146 pagesSelected ES and EHS Studies-2018 by ES-UKJessica Learmond-CriquiNo ratings yet
- Strahlenfolter - Mind Control FAQ BioAPI Gangstalking V2K - Www-Dataasylum-ComDocument3 pagesStrahlenfolter - Mind Control FAQ BioAPI Gangstalking V2K - Www-Dataasylum-ComEmil-Wendtland100% (2)
- EXCLUSIVE Transcripts of Whistleblower Testimonies As Targeted Individuals of U.S. Sponsored Mind Control and Related Hearings and Lectures, August 19, 2016Document268 pagesEXCLUSIVE Transcripts of Whistleblower Testimonies As Targeted Individuals of U.S. Sponsored Mind Control and Related Hearings and Lectures, August 19, 2016Stan J. CaterboneNo ratings yet
- General Information On Skin ParasitesDocument8 pagesGeneral Information On Skin ParasitesQ-Based60% (5)
- Strahlenfolter - Elisa Lam - Targeted Individuals - Based Mind Controlled SlaveDocument38 pagesStrahlenfolter - Elisa Lam - Targeted Individuals - Based Mind Controlled SlaveFred_BlankeNo ratings yet
- Strahlenfolter - Mind Control - Electronic Harassment - WWW - Us-Government-TortureDocument16 pagesStrahlenfolter - Mind Control - Electronic Harassment - WWW - Us-Government-Torturenwo-mengele-doctorsNo ratings yet
- Chemtrails - Bio-Active Crystalline Cationic Polymers From Mike CastleDocument2 pagesChemtrails - Bio-Active Crystalline Cationic Polymers From Mike CastlesisterrosettaNo ratings yet
- Bob Boyce - Un-Requested VeriChip and Associated Tumor Removed - Targeted IndividualsDocument26 pagesBob Boyce - Un-Requested VeriChip and Associated Tumor Removed - Targeted IndividualsTortured-Targeted-IndividualsNo ratings yet
- Is Your Body Infested With Self-Replicating Nano-FibersDocument4 pagesIs Your Body Infested With Self-Replicating Nano-Fibersthisdraft100% (2)
- Dangers of 5G & The Best Crystals For EMF ProtectionDocument15 pagesDangers of 5G & The Best Crystals For EMF ProtectionDMDO100% (1)
- Strahlenfolter Stalking - TI - V2K - RNM Remote Neural Monitoring Satellite Terrorism - October 2010 - Satelliteterrorism3.Blogspot - deDocument5 pagesStrahlenfolter Stalking - TI - V2K - RNM Remote Neural Monitoring Satellite Terrorism - October 2010 - Satelliteterrorism3.Blogspot - deKurt-Schneider100% (1)
- GANGSTALKINGDocument3 pagesGANGSTALKINGGeneration GenerationNo ratings yet
- Bioterrorism and Intelligence PDFDocument11 pagesBioterrorism and Intelligence PDFIbs Júnior100% (1)
- Targeted Individuals Canada - Organized Stalking, Electronic Psychotronic Harassment, Mind ControlDocument18 pagesTargeted Individuals Canada - Organized Stalking, Electronic Psychotronic Harassment, Mind ControlAndreas_PietrzakNo ratings yet
- Strahlenfolter Stalking - TI - Martina Cable - BLOG - TARGETED INDIVIDUALS - EMF SHIELDINGDocument304 pagesStrahlenfolter Stalking - TI - Martina Cable - BLOG - TARGETED INDIVIDUALS - EMF SHIELDINGParanoia-war-gesternNo ratings yet
- 20210219-Mr G. H. Schorel-Hlavka O.W.B. To PM MR SCOTT MORRISON & Ors-Re Vaccination Issues-Suppl 1Document50 pages20210219-Mr G. H. Schorel-Hlavka O.W.B. To PM MR SCOTT MORRISON & Ors-Re Vaccination Issues-Suppl 1Gerrit Hendrik Schorel-HlavkaNo ratings yet
- Morgellons and The CIA's MK - NAOMI Project (Part 2)Document23 pagesMorgellons and The CIA's MK - NAOMI Project (Part 2)scubawoman100% (5)
- Mo'Nique Blacklisted/Blackballed or Targeted Individual 11th ED, February 28th, 2021Document5 pagesMo'Nique Blacklisted/Blackballed or Targeted Individual 11th ED, February 28th, 2021JusticeTINo ratings yet
- Emerging Tech: How Smart Dust Could Revolutionize Medicine and Environment MonitoringDocument31 pagesEmerging Tech: How Smart Dust Could Revolutionize Medicine and Environment MonitoringGauravNo ratings yet
- Privacy in A Surveillance SocietyDocument7 pagesPrivacy in A Surveillance SocietyPavithran RajanNo ratings yet
- Strahlenfolter Stalking - TI - Dorothy Burdick - Such Things Are Known - Chapter 10 - Sm4csi - Home.xs4all - NLDocument22 pagesStrahlenfolter Stalking - TI - Dorothy Burdick - Such Things Are Known - Chapter 10 - Sm4csi - Home.xs4all - NLHermann-SchwanneckeNo ratings yet
- Morgellon Fibers Lucferius Moderna-Fibers-2Document20 pagesMorgellon Fibers Lucferius Moderna-Fibers-2On The Path100% (2)
- Delusions May Not Always Be DelusionsDocument2 pagesDelusions May Not Always Be DelusionsVirginia Savely DNPNo ratings yet
- Strahlenfolter Stalking - TI - Robert O. Butner - Account From Mr. Joseph HohlmanDocument16 pagesStrahlenfolter Stalking - TI - Robert O. Butner - Account From Mr. Joseph HohlmanHans-Werner-Gaertner100% (1)
- Strahlenfolter Stalking - TI - Bibliotecapleyades - Net - Brain ZappingDocument20 pagesStrahlenfolter Stalking - TI - Bibliotecapleyades - Net - Brain ZappingHans-Georg-JakobsonNo ratings yet
- Pg. 291 Pgs. 287 293 JW V DOD and State 14 812 DOD Release 2015 04 10 Final Version11Document7 pagesPg. 291 Pgs. 287 293 JW V DOD and State 14 812 DOD Release 2015 04 10 Final Version11John HinderakerNo ratings yet
- Stratospheric Aerosol Injection (SAI)Document1,119 pagesStratospheric Aerosol Injection (SAI)sisterrosettaNo ratings yet
- Netanyahu Considers It A Religious Duty To Hasten The Arrival of The Messiah by Provoking An Eschatological Confrontation by Thierry MeyssanDocument7 pagesNetanyahu Considers It A Religious Duty To Hasten The Arrival of The Messiah by Provoking An Eschatological Confrontation by Thierry MeyssansisterrosettaNo ratings yet
- CNN LIVE EVENT SPECIAL Benghazi Hearings Aired 6 30p-9 00p ET Aired October 22, 2015 - 18 30 ETDocument53 pagesCNN LIVE EVENT SPECIAL Benghazi Hearings Aired 6 30p-9 00p ET Aired October 22, 2015 - 18 30 ETsisterrosettaNo ratings yet
- Man Is On A Quest - To Play God That's Impossible - Weather Warfare - Saturday, Jul 25 Lightning Researcher Elissa Eastvedt Is Crouched Down in An Underground Bunker at The Top of TheDocument21 pagesMan Is On A Quest - To Play God That's Impossible - Weather Warfare - Saturday, Jul 25 Lightning Researcher Elissa Eastvedt Is Crouched Down in An Underground Bunker at The Top of ThesisterrosettaNo ratings yet
- The Messy US Strategy' in Syria - Pepe EscobarDocument4 pagesThe Messy US Strategy' in Syria - Pepe EscobarsisterrosettaNo ratings yet
- West Crying For Refugees With One Eye, Aiming Gun With The Other' - AssadDocument15 pagesWest Crying For Refugees With One Eye, Aiming Gun With The Other' - AssadsisterrosettaNo ratings yet
- Program Activities of The National Science Foundation - 1959Document75 pagesProgram Activities of The National Science Foundation - 1959sisterrosettaNo ratings yet
- Top British Climate Scientist Acknowledges Ongoing Geoengineering InterventionsDocument6 pagesTop British Climate Scientist Acknowledges Ongoing Geoengineering Interventionssisterrosetta100% (1)
- Chem Trail GEO Engineering Patents, Lab AnalysisDocument23 pagesChem Trail GEO Engineering Patents, Lab AnalysissisterrosettaNo ratings yet
- Bacteriological Weapons in The Coming WarDocument23 pagesBacteriological Weapons in The Coming WarsisterrosettaNo ratings yet
- Be Not Faint Hearted Because Failure Seems To Be in Thy WayDocument7 pagesBe Not Faint Hearted Because Failure Seems To Be in Thy WaysisterrosettaNo ratings yet
- NineEleven Methodical Deception - Rebekah Roth Plus A Few More TheoriesDocument13 pagesNineEleven Methodical Deception - Rebekah Roth Plus A Few More Theoriessisterrosetta67% (3)
- Radio and Laser Frequency and Harmonic Test Ranges For The Lucy and HAARP Experiments and Their Application To Atmospheric Methane DestructionDocument18 pagesRadio and Laser Frequency and Harmonic Test Ranges For The Lucy and HAARP Experiments and Their Application To Atmospheric Methane DestructionsisterrosettaNo ratings yet
- Psychiatric Mind Control Admiral Jeremy Mike Boorda Navy Programming Hit Men and Assassins - Arlene TynerDocument116 pagesPsychiatric Mind Control Admiral Jeremy Mike Boorda Navy Programming Hit Men and Assassins - Arlene TynersisterrosettaNo ratings yet
- Aluminum Poisoning of Humanity and Earth's Biota by Clandestine Geoengineering ActivityDocument9 pagesAluminum Poisoning of Humanity and Earth's Biota by Clandestine Geoengineering Activitysisterrosetta0% (1)
- Foreign Minister Sergey Lavrov Address at The Security Council Open Debate On Maintaining International Peace and SecurityDocument3 pagesForeign Minister Sergey Lavrov Address at The Security Council Open Debate On Maintaining International Peace and SecuritysisterrosettaNo ratings yet
- American Dreaming, From G1 To Bilderberg - Pepe EscobarDocument5 pagesAmerican Dreaming, From G1 To Bilderberg - Pepe EscobarsisterrosettaNo ratings yet
- Higgins James ZeroPointTheStoryOf FILM W2013Document53 pagesHiggins James ZeroPointTheStoryOf FILM W2013sisterrosetta50% (2)
- "The More Sinister Directions of Carbon Nanotubes" (As Weapons of War) Richard Alan MillerDocument20 pages"The More Sinister Directions of Carbon Nanotubes" (As Weapons of War) Richard Alan MillersisterrosettaNo ratings yet
- Saudi War On Yemen - Rising Tensions in The Middle East and The Crisis of ImperialismDocument11 pagesSaudi War On Yemen - Rising Tensions in The Middle East and The Crisis of ImperialismsisterrosettaNo ratings yet
- Just Sharing An Edgar Cayce Reading 4 EasterDocument7 pagesJust Sharing An Edgar Cayce Reading 4 EastersisterrosettaNo ratings yet
- ELF Waves Create Disturbances in The Biological Processes of The Body, Activated On A Large Scale Once The Body Has Been Exposed To The Aforementioned Disease-Causing ChemtrailsDocument20 pagesELF Waves Create Disturbances in The Biological Processes of The Body, Activated On A Large Scale Once The Body Has Been Exposed To The Aforementioned Disease-Causing ChemtrailssisterrosettaNo ratings yet
- 212str Extraterrestrial TechnologyDocument212 pages212str Extraterrestrial TechnologysisterrosettaNo ratings yet
- Stratospheric Aerosol Injection (SAI)Document1,119 pagesStratospheric Aerosol Injection (SAI)sisterrosettaNo ratings yet
- Phil Willis MP Chair Sci and Tech Committee UK - Could People Who Were Adversely Affected by Geoengineering Veto or Alter Geoengineering TestsDocument4 pagesPhil Willis MP Chair Sci and Tech Committee UK - Could People Who Were Adversely Affected by Geoengineering Veto or Alter Geoengineering TestssisterrosettaNo ratings yet
- USAF Colonel Steve Wilson Majestic MJ-12 S4 "Sector 4 - "Project Pounce" Area-51 Kissinger Teller XH-75DsDocument388 pagesUSAF Colonel Steve Wilson Majestic MJ-12 S4 "Sector 4 - "Project Pounce" Area-51 Kissinger Teller XH-75Dssisterrosetta100% (3)
- Sky Striping #ChemTrails and How It Might Work (Or Not) A Local Chemist Looks at The Science by David TulisDocument10 pagesSky Striping #ChemTrails and How It Might Work (Or Not) A Local Chemist Looks at The Science by David TulissisterrosettaNo ratings yet
- The Geopolitics of World War IIIDocument7 pagesThe Geopolitics of World War IIIsisterrosettaNo ratings yet
- Two Recent Pepe Escobar Articles August 2014Document6 pagesTwo Recent Pepe Escobar Articles August 2014sisterrosetta100% (1)
- College of Medicine & Health SciencesDocument56 pagesCollege of Medicine & Health SciencesMebratu DemessNo ratings yet
- The Helping Art Clinical Nursing Who - Google SeaDocument1 pageThe Helping Art Clinical Nursing Who - Google Sea26sbn8d4p9No ratings yet
- Do Your Genes Make You A CriminalDocument39 pagesDo Your Genes Make You A CriminalParisha SinghNo ratings yet
- Tushar FinalDocument29 pagesTushar FinalRaj Prixit RathoreNo ratings yet
- Enemies of BeerDocument24 pagesEnemies of Beerpana0048100% (1)
- A Medical Outreach Elective CourseDocument11 pagesA Medical Outreach Elective CourseRobert SmithNo ratings yet
- Pricelist Aleena Skin Clinic KendariDocument2 pagesPricelist Aleena Skin Clinic Kendariwildan acalipha wilkensiaNo ratings yet
- 10 Doh Approved Herbal MedicineDocument32 pages10 Doh Approved Herbal MedicineIsmael JaaniNo ratings yet
- S.P.P.M. MasterDocument126 pagesS.P.P.M. MasterwahyuNo ratings yet
- 1.2.1 Log 1Document3 pages1.2.1 Log 1linuspauling101100% (6)
- Care of A Child With Cardiovascular DysfunctionDocument71 pagesCare of A Child With Cardiovascular DysfunctionMorgan Mitchell100% (1)
- Psychological Impact of COVID-19 Pandemic in The Philippines PDFDocument13 pagesPsychological Impact of COVID-19 Pandemic in The Philippines PDFAndrea KamilleNo ratings yet
- The Costly Business of DiscriminationDocument46 pagesThe Costly Business of DiscriminationCenter for American Progress100% (1)
- Idiopathic Thrombocytopenic PurpuraDocument3 pagesIdiopathic Thrombocytopenic Purpuraproxytia64No ratings yet
- Prepositions of Time ExplainedDocument18 pagesPrepositions of Time ExplainedyuèNo ratings yet
- PIDSR Other DiseasesDocument45 pagesPIDSR Other DiseasesMichelle TuraNo ratings yet
- MDR Guideline Medical Devices LabelingDocument7 pagesMDR Guideline Medical Devices Labelingarade43100% (1)
- K55 MSDSDocument7 pagesK55 MSDSalocNo ratings yet
- Digestive System PowerpointDocument33 pagesDigestive System PowerpointThomas41767% (6)
- 11 - Comfort, Rest and Sleep Copy 6Document28 pages11 - Comfort, Rest and Sleep Copy 6Abdallah AlasalNo ratings yet
- Congenital LaryngomalaciaDocument8 pagesCongenital LaryngomalaciaRettha SigiroNo ratings yet
- 0007PNTDocument11 pages0007PNTPau Lo JakobyNo ratings yet
- Ten Trends To Watch in The Coming Year - The EconomistDocument1 pageTen Trends To Watch in The Coming Year - The EconomistAnonymous KaoLHAkt100% (1)
- Topical Agents and Dressings For Local Burn Wound CareDocument25 pagesTopical Agents and Dressings For Local Burn Wound CareViresh Upase Roll No 130. / 8th termNo ratings yet
- HSE List of PublicationsDocument12 pagesHSE List of PublicationsDanijel PindrićNo ratings yet
- 380 Final PaperDocument46 pages380 Final Paperapi-538048965No ratings yet
- Journal Homepage: - : IntroductionDocument3 pagesJournal Homepage: - : IntroductionIJAR JOURNALNo ratings yet
- Posthumus 2021 Competition Nutrition Practices ofDocument13 pagesPosthumus 2021 Competition Nutrition Practices ofSyazmi MohdNo ratings yet
- Behavorial Methods IIDocument18 pagesBehavorial Methods IImehak727No ratings yet
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (402)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (327)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (13)
- The Ultimate Guide To Memory Improvement TechniquesFrom EverandThe Ultimate Guide To Memory Improvement TechniquesRating: 5 out of 5 stars5/5 (34)
- Techniques Exercises And Tricks For Memory ImprovementFrom EverandTechniques Exercises And Tricks For Memory ImprovementRating: 4.5 out of 5 stars4.5/5 (40)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsNo ratings yet
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 5 out of 5 stars5/5 (3)
- The Happiness Trap: How to Stop Struggling and Start LivingFrom EverandThe Happiness Trap: How to Stop Struggling and Start LivingRating: 4 out of 5 stars4/5 (1)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (4)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (31)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsFrom EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNo ratings yet
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (41)
- The Tennis Partner: A Doctor's Story of Friendship and LossFrom EverandThe Tennis Partner: A Doctor's Story of Friendship and LossRating: 4.5 out of 5 stars4.5/5 (4)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisFrom EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisRating: 5 out of 5 stars5/5 (3)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisFrom EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisRating: 5 out of 5 stars5/5 (8)