Professional Documents
Culture Documents
Organic Chemistry Klein CH 16 Slides
Uploaded by
Sarah AlexanderOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry Klein CH 16 Slides
Uploaded by
Sarah AlexanderCopyright:
Available Formats
SpecLropscopy lnvolves measuremenLs of lnLeracuon beLween llghL energy and
mauer. We focus on C and P, because Lhese are Lhe elemenLs found ln all organlc
compounds.
1
A magneuc momenL creaLes a magneuc eld.
2
3
lf Lhe LoLal number of proLons and neuLrons ls even, Lhen Lhe aLom wlll noL have a
magneuc momenL aL all, and lL wlll noL be aecLed by Lhe exLernal magneuc eld.
4
lL ls more sLable for Lhe nucleus Lo allgn wlLh Lhe magneuc eld.
3 6
When Lhe magneuc eld sLrengLh ls less, (because lL has been canceled or reduced by
Lhe magneuc eld of Lhe clrculaung elecLrons) Lhere ls a smaller energy gap
7
1he sLrucLure LhaL Lhe molecule ls connecLed wlLh deLermlnes Lhe elecLronlc
envlronmenL for each aLom and Lhus whaL energy gap wlll be observed for each aLom
ln Lhe magneuc eld. So, lf cerLaln energy gaps are observed, concluslons can be
made abouL Lhe sLrucLure of Lhe molecule.
8
9 10
11
ueuLeraLed solvenLs are necessary, because a solvenL wlLh any number of proLons
belng ln large excess would glve a huge slgnal. Slgnals for Lhe soluLe ln comparlson
would be lndlsungulshable above Lhe nolse ln Lhe slgnal.
12
13 14
13 16
17
8ecause of Lhe chlral cenLer LhaL Lhe CP group ls auached Lo, lf one of Lhe labeled P
ls replaced wlLh a u and Lhen Lhe oLher labeled P aLom ls replaced wlLh a u, Lhe
resulung compounds wlll be dlasLereomers. 8esulung dlasLereomers shown above
18
19 20
21
Axlal and equlLorlal proLons have dlerenL elecLronlc envlronmenLs. lf Lhe chalr were
noL lnLerconverung, Lwo slgnals would be observed.
22
lf Lhe LemperaLure were lowered, and Lhe lnLerconverslon were slowed down, Lhen
Lwo slgnals would be observed.
23 24
23 26
27 28
LlecLronegauve aLoms auracL elecLrons away from Lhe P aLoms Lhrough lnducuon.
WlLh fewer elecLrons around Lhe h aLom, Lhe exLernal magneuc eld aecLs Lhe P
aLom more causlng a larger energy gap beLween alpha and beLa spln sLaLes. 1haL
means a greaLer energy wlll be necessary Lo puL Lhe P aLom ln resonance, and Lhe
slgnal wlll appear on Lhe hlgh energy or downeld slde of Lhe specLra.
29 30
31 32
33 34
Some reglons are more shlelded Lhan oLhers, because Lhe elecLron denslLy around
LhaL area ls greaLer, can be aecLed by Lhe shapes of orblLals and by Lhe presence of
pl bonds versus slgma bonds, and Lhe proxlmlLy Lo elecLronegauve aLoms.
33
1he nonequlvalenL P aLoms on Lhe rlng (should see Lhree slgnals) are shled Lo very
slmllar posluons, so Lhey overlap creaung a slgnal LhaL appears as lf lL ls one slgnal.
36
1he P aLoms on Lhe ouLslde of Lhe sLrucLure appear aL 8 ppm, whlle Lhe proLons
polnung lnLo Lhe pl cloud appear around -1 ppm.
37 38
39 40
41
1erL-buLyl meLhyl eLher would glve Lwo slgnals. Cne slgnal for Lhe meLhyl group aL
approxlmaLely 3.3 ppm wlLh an lnLegrauon of 3. 1he oLher slgnal for Lhe LerL-buLyl
group, whlch has Lhree equlvalenL meLhyl groups. lLs shl would be approxlmaLely 1
ppm wlLh an lnLegrauon of 9.
3-penLanone would have Lwo slgnals because of symmeLry. Cne slgnal for Lhe Lwo
CP2 groups on elLher slde of Lhe carbonyl wlLh a shl of approxlmaLely 2.2 ppm and
an lnLegrauon of 4. 1he oLher slgnal would shl Lo approxlmaLely 1 ppm wlLh an
lnLegrauon of 6.
42
43 44
43 46
When one nelghborlng proLon ls shleldlng and Lhe oLher ls deshleldlng, Lhe eecLs
cancel ouL.
47
1he LrlpleL resulLs when one nelghborlng proLon shlelds and Lhe oLher deshlelds half
Lhe ume (glvlng Lhe cenLer peak wlLh a lnLegrauon Lwlce LhaL of Lhe ouLer peaks).
48
nelghborlng P aLoms LhaL allgn Lhelr spln wlLh Lhe magneuc eld have dlerenL
shleldlng eecLs Lhan nelghborlng P aLoms LhaL allgn agalnsL Lhe magneuc eld.
49
lnLegrauon rauos should be 1:3:3:1
30
31
n+1 rule means Lhe spllmng should be equal Lo Lhe number of P aLoms on Lhe
ad[acenL carbon +1. Lxplalned more Lhrough examples on subsequenL slldes.
32
1,2-dlchloroeLhane should be a slngleL
33 34
33 36
37 38
39 60
61
lf nonequlvalenL P aLoms spllL wlLh Lhe same coupllng consLanL, Lhen Lhey acL as lf
Lhey are equlvalenL, and Lhe spllmng pauern wlll follow Lhe n+1 rule. See analysls for
(!)-penL-2-en-4-ol on followlng sllde.
62
lf nonequlvalenL P aLoms spllL wlLh Lhe same coupllng consLanL, Lhen Lhey acL as lf
Lhey are equlvalenL, and Lhe spllmng pauern wlll follow Lhe n+1 rule. See analysls for
(!)-penL-2-en-4-ol on followlng sllde.
63 64
Mechanlsm on nexL sllde
63 66
1he solvenL wlll be ln a large excess, so Lhe equlllbrlum creaLed where someumes Lhe
molecule has a P aLom and someumes a u aLom wlll favor replacemenL of Lhe P aLom
wlLh u aLoms.
67 68
Lach molecule has slmllar funcuonal groups, so Lhe sLreLchlng vlbrauons wlll be very
slmllar pracucally lndlsungulshable. 1he proLon nM8 for each would glve a dlerenL
number of slgnals (from le Lo rlghL 3 slgnals, 4 slgnals, 2 slgnals).
69
1he rlghL hand slde of Lhe molecule ls Lhe same for boLh, buL on Lhe le hand slde
oLher Lhe molecule, Lhe spllmng pauers wlll be dlerenL. Movlng from le Lo rlghL,
Lhe molecule on Lhe le has a doubleL, quarLeL, slngleL, eLc. 1he molecule on Lhe
rlghL has a LrlpleL, quarLeL, slngleL, eLc.
70
71
Pul = 1. 1he slngleL lnLegraung Lo 6P suggesLs Lwo meLhyl groups.
72
1he doubleL of quarLeLs shled Lo 3.3 ppm suggesLs a CP auached Lo a C=C double
bond wlLh a meLhyl group auached Lo Lhe carbon and a slngle P on Lhe oLher carbon
of Lhe C=C double bond. 1he peak aL 3.4 ppm suggesLs LhaL Lhere ls a carbon wlLh 2
P aLoms auached Lo Lhe Cl and nexL Lo a carbon wlLh no P aLoms on lL. 1he molecule
works ouL Lo be cls or Lrans 3-chloro-4,4-dlmeLhyl-2-penLene.
73 74
73 76
2 slgnals
77 78
79 80
81
ln Lhe molecule on Lhe le, Lhe CP2 ls shled farLhesL downeld. ln Lhe molecule on
Lhe rlghL, Lhe CP ls shled farLhesL downeld, and ln uL1 CP and CP2 are
dlsungulshable.
82
Some ussues have more waLer versus oLher molecules LhaL may have more carbon or
oLher elemenLs. A 3u lmage resulLs from Lhe dlerenL slgnals glven ln dlerenL
ussues. no known slde eecLs Lo radlo waves or magneuc elds.
83
You might also like
- Electronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestFrom EverandElectronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestNo ratings yet
- Analy&cal Methods in Chemistry (CH - 429)Document58 pagesAnaly&cal Methods in Chemistry (CH - 429)Surender MalikNo ratings yet
- Organic Chemistry Study Guide: Key Concepts, Problems, and SolutionsFrom EverandOrganic Chemistry Study Guide: Key Concepts, Problems, and SolutionsRating: 3.5 out of 5 stars3.5/5 (10)
- Organic Chemistry Klein Chapter 20 PowerPointDocument36 pagesOrganic Chemistry Klein Chapter 20 PowerPointSarah AlexanderNo ratings yet
- Thermodynamic Properties of MethanolDocument24 pagesThermodynamic Properties of MethanolJessica FernandesNo ratings yet
- Year 10 Science Yearly NotesDocument27 pagesYear 10 Science Yearly Notesunstoppable1234No ratings yet
- Engineering-Related Concepts and Associated MathematicsDocument25 pagesEngineering-Related Concepts and Associated Mathematicschesspalace2No ratings yet
- Bioelectricity Week 1 Slides Feb 2013Document94 pagesBioelectricity Week 1 Slides Feb 2013darkneslifeNo ratings yet
- Resonance Interactions in Acyclic Systems: IupacDocument8 pagesResonance Interactions in Acyclic Systems: IupacAmOo ChurailNo ratings yet
- CM1502 Chapter 2 - 2013-14Document40 pagesCM1502 Chapter 2 - 2013-14Minh TieuNo ratings yet
- ACS Biochemistry Study PrepDocument12 pagesACS Biochemistry Study PrepDanny Boyette100% (2)
- DR John Wilkie NotesDocument32 pagesDR John Wilkie NotesSyed Azaam QadriNo ratings yet
- Molecular Orbital Theory From Concise Inorganic Chemistry by J.DDocument31 pagesMolecular Orbital Theory From Concise Inorganic Chemistry by J.DJJJJ TarkaNo ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 11Document58 pagesVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmNo ratings yet
- Exercise 4 IR+UV Calculations: 4.1 Infrared AbsorbtionDocument6 pagesExercise 4 IR+UV Calculations: 4.1 Infrared AbsorbtionSanne Maassen van den BrinkNo ratings yet
- Electronic StructureDocument3 pagesElectronic Structuremasten.gregoryNo ratings yet
- CLEDHI PosterDocument1 pageCLEDHI PosterLauren BiddleNo ratings yet
- Spectroscopy ManualDocument21 pagesSpectroscopy Manualanthor100% (2)
- Lecture Notes On Quantum Mechanics Multi Electron SystemsDocument14 pagesLecture Notes On Quantum Mechanics Multi Electron Systemsliv2luvNo ratings yet
- AdsorptionDocument20 pagesAdsorptionCheska BiolenaNo ratings yet
- Molecular Cell Biology Revision NotesDocument13 pagesMolecular Cell Biology Revision NotesRPh Krishna Chandra JagritNo ratings yet
- ProjectDocument21 pagesProjectHitesh PandeyNo ratings yet
- Goljan ImpDocument246 pagesGoljan ImpShri ReddyNo ratings yet
- Garay Midterm Review Sheet 2011 2012-2Document23 pagesGaray Midterm Review Sheet 2011 2012-2api-241577069No ratings yet
- Dye Solar Cell Chem4B SP14Document11 pagesDye Solar Cell Chem4B SP14peterbishop89No ratings yet
- Prep 101 Booklet (2013) Part 2Document24 pagesPrep 101 Booklet (2013) Part 2Alexandre SaymanNo ratings yet
- Alkenes and AlkynesDocument39 pagesAlkenes and Alkynesrajeevbansal1807No ratings yet
- Orbital Symmetry Lecture For StudentDocument101 pagesOrbital Symmetry Lecture For StudentEmil Ranjan DasNo ratings yet
- Atomic Structure: Protons, Neutrons and ElectronsDocument3 pagesAtomic Structure: Protons, Neutrons and Electronsmasten.gregoryNo ratings yet
- EMC Tutorial QuestionsDocument5 pagesEMC Tutorial QuestionsAli DurazNo ratings yet
- 569 Pages, Chapter 15.3-23.10Document569 pages569 Pages, Chapter 15.3-23.10SanyaNo ratings yet
- Chemistry-II Course Code: CHM-3135: Saiful Islam SohagDocument13 pagesChemistry-II Course Code: CHM-3135: Saiful Islam SohagABID HASASNNo ratings yet
- H2 Chemistry NotesDocument9 pagesH2 Chemistry NotescsngNo ratings yet
- NMR N M R: Uclear Agnetic EsonanceDocument33 pagesNMR N M R: Uclear Agnetic EsonanceCarolina AlpucheNo ratings yet
- Chapter 3Document11 pagesChapter 3Marianna UcedaNo ratings yet
- Assignment 1Document4 pagesAssignment 1Yamer YusufNo ratings yet
- NMR SpectrumDocument5 pagesNMR SpectrumNabaa KhaldoonNo ratings yet
- Advanced Organic Chemistry-Wim DehaenDocument230 pagesAdvanced Organic Chemistry-Wim DehaenNguyenRingNo ratings yet
- Unit 4Document63 pagesUnit 4api-268467602No ratings yet
- Class 5: Exano F Bizi. Imen o Hyid inDocument40 pagesClass 5: Exano F Bizi. Imen o Hyid inUnknown IdNo ratings yet
- Cie Structured Quiz Practice AnswersDocument7 pagesCie Structured Quiz Practice AnswersSahanNivanthaNo ratings yet
- Absorption Spectrum of A Conjugated DyeDocument6 pagesAbsorption Spectrum of A Conjugated DyeKing Everest100% (1)
- Chapter 3Document38 pagesChapter 3민규강No ratings yet
- Thermal DesorptionDocument2 pagesThermal DesorptionSai HtweNo ratings yet
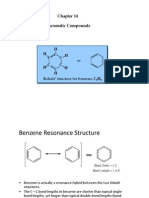
- Aromatic Compounds: C C C C C C H H H orDocument35 pagesAromatic Compounds: C C C C C C H H H orJohn SmithNo ratings yet
- ChemPhysics MCAT NotesDocument14 pagesChemPhysics MCAT NotesChris HuebnerNo ratings yet
- Alkenes and AlkynesDocument85 pagesAlkenes and AlkynesAdel M MalkawiNo ratings yet
- Chemistry 12Document13 pagesChemistry 12Asajnd LJAsnNNo ratings yet
- Molarity, Molality and NormalityDocument6 pagesMolarity, Molality and NormalitySami FlimbanNo ratings yet
- Chapter 11 Ligand SubstitutionDocument20 pagesChapter 11 Ligand SubstitutionahmedkhidryagoubNo ratings yet
- Organic Chemistry 1 NotesDocument27 pagesOrganic Chemistry 1 Noteszeeshan_haider000No ratings yet
- Chapter7elimination Ans SubstnDocument22 pagesChapter7elimination Ans Substnjagabandhu_patraNo ratings yet
- Chemistry PryctDocument9 pagesChemistry PryctAlejandra G.CabanillasNo ratings yet
- C - NMR: Cracking Those Carbons: Parallel, and Those With Axes Pointing in Opposite Directions Are Called AntiparallelDocument6 pagesC - NMR: Cracking Those Carbons: Parallel, and Those With Axes Pointing in Opposite Directions Are Called AntiparallelMusdalifahNo ratings yet
- MCAT Organic Summary SheetDocument6 pagesMCAT Organic Summary SheetSpencer Thomas100% (2)
- Alkenes Infrared Spectroscopy and Mass SpectrosDocument40 pagesAlkenes Infrared Spectroscopy and Mass Spectrosalexandra owNo ratings yet
- Alkenes AlkynesDocument40 pagesAlkenes AlkynesHanindya NugrahaNo ratings yet
- Ch20 Sec1to2 Lecture-PpDocument8 pagesCh20 Sec1to2 Lecture-PpaiudfuhNo ratings yet
- Intro To Comp SimulationDocument14 pagesIntro To Comp SimulationMudit MisraNo ratings yet
- Ayaan Hirsi Ali - Paper For Black DanceDocument6 pagesAyaan Hirsi Ali - Paper For Black DanceSarah AlexanderNo ratings yet
- Organic Synthesis of VanillinDocument6 pagesOrganic Synthesis of VanillinSarah Alexander0% (1)
- Twelve Days of Christmas Piano Sheet Music With LyircsDocument3 pagesTwelve Days of Christmas Piano Sheet Music With LyircsSarah Alexander100% (1)
- Science WarsDocument2 pagesScience WarsSarah AlexanderNo ratings yet
- EDU 280 Family History ReportDocument2 pagesEDU 280 Family History ReportSarah AlexanderNo ratings yet
- Cognitive Psychology Chapter 4Document11 pagesCognitive Psychology Chapter 4Sarah AlexanderNo ratings yet
- Chapter 6: Educational PsychologyDocument12 pagesChapter 6: Educational PsychologySarah AlexanderNo ratings yet
- Synthesis of Ferrocenylenones Via Claisen Condensation Under UltrasonicationDocument9 pagesSynthesis of Ferrocenylenones Via Claisen Condensation Under UltrasonicationSarah AlexanderNo ratings yet
- Sexism in ScienceDocument2 pagesSexism in ScienceSarah AlexanderNo ratings yet
- Psychology of EducationDocument10 pagesPsychology of EducationSarah AlexanderNo ratings yet
- Chapter9 Problems Solutions Physical Chem AtkinsDocument10 pagesChapter9 Problems Solutions Physical Chem AtkinsSarah AlexanderNo ratings yet
- Chem 422 (Physical Chem) Quiz 3Document1 pageChem 422 (Physical Chem) Quiz 3Sarah AlexanderNo ratings yet
- Physical Chemistry 2 Week 1Document46 pagesPhysical Chemistry 2 Week 1Sarah AlexanderNo ratings yet
- Physical Chemistry Chapter 1 LectureDocument9 pagesPhysical Chemistry Chapter 1 LectureSarah AlexanderNo ratings yet
- Theory Success and Failure On Silver MonohalidesDocument14 pagesTheory Success and Failure On Silver MonohalidesSarah AlexanderNo ratings yet
- Recipe For Vegan Banana Chocolate Chip MuffinsDocument1 pageRecipe For Vegan Banana Chocolate Chip MuffinsSarah AlexanderNo ratings yet
- Sigma Bond - Wikipedia, The Free EncyclopediaDocument2 pagesSigma Bond - Wikipedia, The Free EncyclopediaAlamgir Kabir ShuvoNo ratings yet
- Molecular Orbital Theory JCDocument13 pagesMolecular Orbital Theory JCSaikhom TutorsNo ratings yet
- Calibration of UvDocument3 pagesCalibration of UvChilaNo ratings yet
- Finals Pharmaceutical AnalysisDocument13 pagesFinals Pharmaceutical Analysistayyab malik100% (1)
- Hybridization Notes HDocument3 pagesHybridization Notes HArian RayaNo ratings yet
- Combination Bands, Overtones and Fermi ResonancesDocument5 pagesCombination Bands, Overtones and Fermi ResonancesSaurav PaulNo ratings yet
- Identifying Elements Through Spectral Analysis: Andrew SpinelliDocument5 pagesIdentifying Elements Through Spectral Analysis: Andrew SpinelliAndrew SpinelliNo ratings yet
- Free Ion Term ExplanationDocument18 pagesFree Ion Term ExplanationskhjghwudosndgkNo ratings yet
- Problem Set 3Document3 pagesProblem Set 3Aya HachanaNo ratings yet
- DeGroot - Models For XAS and XMCDDocument94 pagesDeGroot - Models For XAS and XMCDbuddlightbeerlogNo ratings yet
- Nonlinear DynamicsDocument374 pagesNonlinear DynamicsmystekxNo ratings yet
- Photoelectron Spectroscopy Worksheet-KEYDocument3 pagesPhotoelectron Spectroscopy Worksheet-KEYAriel NogalesNo ratings yet
- Basis Set: Harno D Pranowo Pusat Kimia Komputasi Indonesia-Austria Fmipa UgmDocument16 pagesBasis Set: Harno D Pranowo Pusat Kimia Komputasi Indonesia-Austria Fmipa UgmNdhoz Los GandhozNo ratings yet
- Hydrothermal Microwave - A New Route To Obtain Photoluminescent Crystalline BaTiO NanoparticlesDocument9 pagesHydrothermal Microwave - A New Route To Obtain Photoluminescent Crystalline BaTiO NanoparticlesDaniel Carvalho de AraújoNo ratings yet
- CHAPTER 2-MOLECULAR ABSORPTION SPECTROSCOPY - Part 4Document59 pagesCHAPTER 2-MOLECULAR ABSORPTION SPECTROSCOPY - Part 4fatin harrisNo ratings yet
- Basic Principle of IR Spectroscopy or IR AbsorptionDocument10 pagesBasic Principle of IR Spectroscopy or IR AbsorptionTanveer HussainNo ratings yet
- 2155 9872 S11 001Document8 pages2155 9872 S11 001Aniket MahapureNo ratings yet
- AnskjhbljsDocument7 pagesAnskjhbljsmillinagi95No ratings yet
- Fluorescence WikiDocument9 pagesFluorescence WikiPalla MadhuNo ratings yet
- VBT & MotDocument15 pagesVBT & MotJaydeep Deore100% (1)
- Exercise - Valence Bond MethodDocument1 pageExercise - Valence Bond MethodHạ Thi LêNo ratings yet
- High Spin and Low SpinDocument13 pagesHigh Spin and Low SpinAyanChatterjeeNo ratings yet
- Legal Foundation of EducationDocument3 pagesLegal Foundation of EducationKatherine Doroja Mariano100% (1)
- Photocatalysis - IV: Lect. 12Document11 pagesPhotocatalysis - IV: Lect. 12Hadeer AbdelazizNo ratings yet
- CHM 213 - Final Exam - 2Q2017-18Document4 pagesCHM 213 - Final Exam - 2Q2017-18cfmonarquia100% (1)
- Paschoal Et Al. 2016Document60 pagesPaschoal Et Al. 2016A1234 AJEFNo ratings yet
- Physics Sliptest SpectrosDocument9 pagesPhysics Sliptest SpectrosJagan EashwarNo ratings yet
- Calibration User GuideDocument8 pagesCalibration User GuideJorge OrtegaNo ratings yet
- CFTDocument15 pagesCFTGaurav BothraNo ratings yet
- Fluorescnec 1Document21 pagesFluorescnec 1vscdNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Fundamentals of Chemistry: A Modern IntroductionFrom EverandFundamentals of Chemistry: A Modern IntroductionRating: 5 out of 5 stars5/5 (1)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- High School Chemistry: Comprehensive Content for High School ChemistryFrom EverandHigh School Chemistry: Comprehensive Content for High School ChemistryNo ratings yet
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet