Professional Documents
Culture Documents
Leaching Info
Uploaded by
Christer John UyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Leaching Info
Uploaded by
Christer John UyCopyright:
Available Formats
Coulson and Richardson Leaching Leaching is concerned with the extraction of a soluble constituent from a solid by means of a solvent.
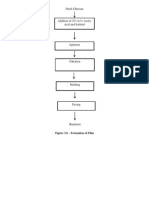
In leaching, soluble material is dissolved from its mixture with an inert solid by means of a liquid solvent. (McCabe) {Explanation may be: leaching is primarily concerned with the extraction of a soluble constituents by means of a solvent.} {The process may be used either for the production of a concentrated solution of a valuable solid material, or in order to remove an insoluble solid, such as a pigment, from a soluble material with which it is contaminated.} The method used for the extraction is determined by the proportion of soluble constituent present, its distribution throughout the solid, the nature of the solid and the particle size. {In comparing leaching with other processes...} In leaching, the amount of soluble material removed is often greater than in ordinary filtration washing, and the properties of the solids may change considerably during the leaching operation. (McCabe) Processes involved Three distinct processes are usually involved in leaching operations: (a) Dissolving the soluble constituent. (b) Separating the solution, so formed, from the insoluble solid residue. (c) Washing the solid residue in order to free it of unwanted soluble matter or to obtain as much of the soluble material as possible as the product.
There are four important factors to be considered: Particle size. Particle size influences the extraction rate in a number of ways. The smaller the size, the greater is the interfacial area between the solid and liquid, and therefore the higher is the rate of transfer of material and the smaller is the distance the solute must diffuse within the solid as already indicated. On the other hand, the surface may not be so effectively used with a very fine material if circulation of the liquid is impeded, and separation of the particles from the liquid and drainage of the solid residue are made more difficult. It is generally desirable that the range of particle size should be small so that each particle requires approximately the same time for extraction and, in particular, the production of a large amount of fine material should be avoided as this may wedge in the interstices of the larger particles and impede the flow of the solvent. Solvent. The liquid chosen should be a good selective solvent and its viscosity should be sufficiently low for it to circulate freely. Generally, a relatively pure solvent will be used
initially, although as the extraction proceeds the concentration of solute will increase and the rate of extraction will progressively decrease, first because the concentration gradient will be reduced, and secondly because the solution will generally become more viscous. Temperature. In most cases, the solubility of the material which is being extracted will increase with temperature to give a higher rate of extraction. Further, the diffusion coefficient will be expected to increase with rise in temperature and this will also improve the rate of extraction. In some cases, the upper limit of temperature is determined by secondary considerations, such as, for example, the necessity to avoid enzyme action during the extraction of sugar. Agitation of the fluid. Agitation of the solvent is important because this increases the eddy diffusion and therefore the transfer of material from the surface of the particles to the bulk of the solution, as discussed in the following section. Further, agitation of suspensions of fine particles prevents sedimentation and more effective use is made of the interfacial surface.
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Gaseous FuelDocument12 pagesGaseous FuelChrister John UyNo ratings yet
- Gaseous FuelDocument12 pagesGaseous FuelChrister John UyNo ratings yet
- Calibration of Acid BuretteDocument7 pagesCalibration of Acid BuretteChrister John Uy100% (2)
- Seawater Quality Assessment and Identification of Pollution Sources Along the Central Coastal Area of Gabes Gulf (SE Tunisia)_ Evidence of Industrial Impact and Implications for Marine Environment ProtectionDocument8 pagesSeawater Quality Assessment and Identification of Pollution Sources Along the Central Coastal Area of Gabes Gulf (SE Tunisia)_ Evidence of Industrial Impact and Implications for Marine Environment ProtectionChrister John UyNo ratings yet
- Abstract UyDocument1 pageAbstract UyChrister John UyNo ratings yet
- Ideal Solns, Colig PropsDocument19 pagesIdeal Solns, Colig PropsChrister John Uy50% (2)
- Second SOC of Guimaras Province (20181205) Smaller Opt PDFDocument226 pagesSecond SOC of Guimaras Province (20181205) Smaller Opt PDFChrister John UyNo ratings yet
- The University Wit To Win ItDocument2 pagesThe University Wit To Win ItChrister John UyNo ratings yet
- Recrystallization & Melting Point Determination Purification of Crystalline Organic CompoundDocument10 pagesRecrystallization & Melting Point Determination Purification of Crystalline Organic CompoundChrister John UyNo ratings yet
- Wetlands: Prepared By: Benedict J. Salazar Christer John M. UyDocument30 pagesWetlands: Prepared By: Benedict J. Salazar Christer John M. UyChrister John UyNo ratings yet
- Op-Ed: Dolphin For Dinner? Poor Countries Increasing Dolphin Consumption Elizabeth Batt, January 8, 2012 SummaryDocument3 pagesOp-Ed: Dolphin For Dinner? Poor Countries Increasing Dolphin Consumption Elizabeth Batt, January 8, 2012 SummaryChrister John UyNo ratings yet
- D3300 SG (En) 02Document144 pagesD3300 SG (En) 02Christer John UyNo ratings yet
- Thermo ProblemsDocument8 pagesThermo ProblemsChrister John UyNo ratings yet
- Potentiometer: Schematic SymbolDocument1 pagePotentiometer: Schematic SymbolChrister John UyNo ratings yet
- Registration Form (Interbarkada)Document2 pagesRegistration Form (Interbarkada)Christer John UyNo ratings yet
- Figure 3.6Document1 pageFigure 3.6Christer John UyNo ratings yet
- Change Does Not Roll in On The Wheels of InevitabilityDocument2 pagesChange Does Not Roll in On The Wheels of InevitabilityChrister John UyNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- MBC21 JNotesDocument77 pagesMBC21 JNotesAbisai Maringe AbbieNo ratings yet
- DPM FluentDocument182 pagesDPM Fluentபார்த்தசாரதி சுப்ரமணியன்No ratings yet
- Lidocaine - HPLCDocument3 pagesLidocaine - HPLCRoger (Sisfarma)No ratings yet
- MRI Lecture NotesDocument33 pagesMRI Lecture NotesArungoud PoshalaNo ratings yet
- Welding Defect - WikipediaDocument7 pagesWelding Defect - WikipediaKiky IchanafiNo ratings yet
- Fuel CellDocument27 pagesFuel CellGallium TNo ratings yet
- 06 Train Track Dynamics June 08Document21 pages06 Train Track Dynamics June 08Charles KohNo ratings yet
- 2 VaDocument48 pages2 VamtttusharNo ratings yet
- Rotameter Overview PDFDocument8 pagesRotameter Overview PDFSteve Goke AyeniNo ratings yet
- Physics Chapter 23 SolutionsDocument15 pagesPhysics Chapter 23 Solutionsalkalkindin119284849No ratings yet
- Slides - Chapter 09Document88 pagesSlides - Chapter 09Mohammad ShawqiNo ratings yet
- Photonics Essentials An Introduction With Experiments 2003Document284 pagesPhotonics Essentials An Introduction With Experiments 2003Kajari Chatterjee100% (1)
- Jablonski DiagramDocument6 pagesJablonski DiagramLipsa PradhanNo ratings yet
- Equilibrium of Rigid BodiesDocument22 pagesEquilibrium of Rigid BodiesSpiro DourbalyNo ratings yet
- S.MORRIS 2006: This Powerpoint Is Hosted On Please Visit For 100's More Free PowerpointsDocument20 pagesS.MORRIS 2006: This Powerpoint Is Hosted On Please Visit For 100's More Free PowerpointsDedy SatriyoNo ratings yet
- RheologyDocument33 pagesRheologyLindsey Barber100% (1)
- Vibration Analysis For Hydraulic PumpDocument18 pagesVibration Analysis For Hydraulic PumpManikandanNo ratings yet
- Answer Key: 13 VXY (Date: 12-02-2012) Review Test-7 Paper-2Document18 pagesAnswer Key: 13 VXY (Date: 12-02-2012) Review Test-7 Paper-2vishal110085No ratings yet
- Transport Phenomena ProblemsDocument26 pagesTransport Phenomena ProblemsCamilo SierraNo ratings yet
- Nacela Service Manual h14tx h16tpxDocument272 pagesNacela Service Manual h14tx h16tpxGules GheorgheNo ratings yet
- The Language of Our Dna Scalar EnergyDocument5 pagesThe Language of Our Dna Scalar EnergyNat Silva100% (1)
- Hadron ColliderDocument121 pagesHadron ColliderDeepak VermaNo ratings yet
- Lec2-Qubits and Uncertainity PrincipleDocument16 pagesLec2-Qubits and Uncertainity PrincipleAmarNathHNo ratings yet
- Deposit Control PMsDocument18 pagesDeposit Control PMsMünir KarıncaoğluNo ratings yet
- Physical Science Test: More Than One of The AboveDocument26 pagesPhysical Science Test: More Than One of The AboveYago RiveraNo ratings yet
- Cern HistoryDocument164 pagesCern HistoryMarco PonteNo ratings yet
- Properties of CompoundsDocument15 pagesProperties of CompoundsPrasad YarraNo ratings yet
- Numc PDFDocument18 pagesNumc PDFMadhur MayankNo ratings yet
- Geometric Shapes and TransformationsDocument10 pagesGeometric Shapes and TransformationsisidroNo ratings yet