Professional Documents
Culture Documents
PH Carbonation Reading
Uploaded by
Arun GuptaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PH Carbonation Reading
Uploaded by
Arun GuptaCopyright:
Available Formats
Portables
Isolation Amplifiers Transducers
Laboratory meters
Process Analytics
Indicators
Author: Dr. Dirk Steinmller
Application Report
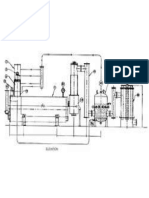
pH Measurements during Carbonatation for Sugar Production
Procedure
Sampling
Beet farm
Extraction Cutting machine T Tower extractor
Pulp drying Pulp presses
Discharging
Beet washing machine
Cossettes
Pulp dryer
Raw juice Juice purification 1st carbonatation Main liming 2nd carbonatation Preliming Thickening White sugar
Pelletizing
Cristallization Cane sugar Post product
Evaporators
Steam
CO2
CO2 Filter Centrifuges Steam Filter T Turbine Thick juice tank Boiler Cooler Sugar conditioning and storage Dryer Sugar silo Molasses
Lime Lime kiln
Carbonatation lime
Filter press Power station
Sensors
Fittings
Portables
Isolation Amplifiers Transducers
Laboratory meters
Process Analytics
Indicators
Application Report Page 2
Background
Today, sugar is one of the most important staple foods of mankind. It is also of enormous economic importance: Sugar is produced in 127 countries. Sugar cane and sugar beets are cultivated on an area of approx. 25 mio hectares worldwide with a sugar production of approx. 120 mio tons. Cane sugar accounts for the major part (2/3) of the sugar produced compared to beet sugar. The largest sugar-producing countries are: India (> 12 Mio t), former USSR, Cuba, Brasil, USA, China, France, Australia, Thailand, Mexico, Germany (4 Mio t), Turkey, Italy, and Poland (2 Mio t). In Germany the per-capita consumption is approx. 35 kg per year.
Procedure
Sugar cane and sugar beet contain up to 20 % sugar (chemical: saccharose). Whether sugar cane or sugar beet serve as source material is only distinguished by different production processes in the beginning, during delivery and cutting, and by different washing procedures. The hackled pieces are leached with 70 C hot water in the so-called diffusion tower. The raw juice produced that way contains almost 99% of the original sugar, however also various organic and inorganic constituents, the so-called non-sugar particles. The juice is purified using lime and carbonic acid. For that purpose, the sugar plants operate lime kilns where lime stone (calcium carbonate) is heated to produce burnt lime (calcium oxide) and carbon dioxide. CaCO3 + heat CaO + CO2
The lime is added to the raw juice as lime milk. In the process, loose calcium hydroxide precipitates are formed which bind the non-sugar particles. CaO + H2O Ca(OH)2
Now, the carbon dioxide is led into this mixture. The lime including the nonsugar particles stably precipitates and can be separated by filtration. Ca(OH)2 + CO2 CaCO3 + H2O
This step is called carbonatation. It is repeated in a second stage. A clear, light-yellow thin juice with a sugar content of approx. 16% remains, which is further processed for thickening. The filtrated carbonated lime is used as fertilizer. The sugar is cleaned by solely physical processes (crystalline transformation and centrifugation). In contrast to widespread assumptions, white sugar is not bleached. Brown sugar only contains more syrup (always brown).
Carbonatation towers
Sensors
Fittings
Portables
Isolation Amplifiers Transducers
Laboratory meters
Process Analytics
Indicators
Application Report Page 3
Measurement and control problems
The efficiency of carbonatation strongly depends on the pH value. The pH value is continuously measured during both carbonatation steps. During the first step, the pH value is kept between 10 and 11. To do so, carbon dioxide (from the lime kiln process) is introduced at approx. 70 C so that the lime precipitates together with the impurities. However, the filter cake from this first carbonatation still contains considerable amounts of sugar and is therefore washed. The sugary wash water is returned to the first stage of the process together with the filtrate. However, it still contains undesired calcium hydroxide. Now the remaining lime is precipitated at 90 95 C in a second carbonatation process. If the pH value falls too much due to the introduced carbon dioxide (carbonic acid in water), the lime decomposes to form hydrogen carbonate. If the value remains too alkaline, the precipitation is incomplete. It is intended to keep the pH around the neutral point at approx. 7.5. To ensure easy comparability with laboratory values, the pH value which is measured at a process temperature of 90 C is automatically recalculated to its 20 C value by smart pH transmitters so that the displayed value is pH 9. The challenge to a pH measuring point in the second carbonatation stage: High content of solids, High temperature (90C) Excessive deposit formation by lime, non-sugar particles, and sticky syrup. During the sugar campaign (in Europe up to 100 days after the harvest of sugar beets in September) these measuring points must be checked and cleaned several times a day.
Solution:
The Ceramat WA 150 sensor lockgate together with the Unical 9000 automatic cleaning and calibration system allows complete automation of this difficult measuring point with maximum availability. The Ceramat lock-gate consists of a virtually undestructible, ultrahard, superpolished, rotating ceramic part and a corrosion resistant, carbon reinforced, nonmoved plastic (PEEK) housing. The ceramic with its rotary movement is not influenced by the incrustations and the static probe housing shows hardly any deposits. In conjunction with the automatic Unical controller, the sensor is automatically cleaned and calibrated at regular intervals. Due to the sluggishness of the process (1 measured value per 30 min), the sensor can stay in the rinsing chamber and just briefly be moved into the process for measurement (e.g. for 10 s every minute) to increase its service life. Cleaning is fully automated using amidosulphonic acid. This system allows fully automated operation of the measurement point during the complete campaign.
Often the pH sensors must be cleaned using an acidic cleaning agent, e.g. amidosulphonic acid. Electrode life is reduced by abrasion and blocking of the reference system. Until today, automation of the cleaning (and calibration) procedure has failed due to the probes and holders used. Metallic ball valve or displacement probes get stuck after a short time thus leading to failure of the pH measurement. Plastic probe holders do not withstand the mechanical and thermal stresses.
Ceramat WA 150 ceramic sensor lock-gate
Ceramic rotary slide
Pneumatic drive Molded housing, non-moved Sensor opening
Sensors
Fittings
Portables
Isolation Amplifiers Transducers
Laboratory meters
Process Analytics
Indicators
Application Report Page 4
Knick Elektronische Messgerte GmbH & Co. KG Beuckestrae 22, 14163 Berlin Phone: +49 (0)30 - 801 91 - 0 Fax: +49 (0)30 - 801 91 - 200 knick@knick.de www.knick.de
The complete measuring system: Highest reliability Optimal process control Low cost of ownership
Protos 3400 (X) analyzer
Unical 9000 (X) controller
Service switch
Ceramat WA 150 sensor lock-gate Cleaning and calibration solutions
pH sensor
Applied Components
Ceramat WA150 N0AAC1-000 Flow-through fitting YF-AR1225 Unical 9000-NC301222CN000-000 Protos 3400C with Unical module PHU 3400-110 pH combination electrode SE 532/2 (225 mm) Sensor cable VP ZU 0314 Buffer solutions pH 4,01 ZU 0200 and pH 7,0 ZU 0201 AB Zucker V1 0305 en
Sensors
Fittings
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Aurduino To ExcelDocument1 pageAurduino To ExcelArun GuptaNo ratings yet
- Wireless Behera 2015Document50 pagesWireless Behera 2015Arun GuptaNo ratings yet
- Limed Juice Flash TankDocument1 pageLimed Juice Flash TankArun GuptaNo ratings yet
- Logix Designer Report(s)Document1 pageLogix Designer Report(s)Arun GuptaNo ratings yet
- Weighing SystemDocument151 pagesWeighing SystemArun GuptaNo ratings yet
- S. N Description QTY Mtrs Unit Price Rs Total Amount RsDocument1 pageS. N Description QTY Mtrs Unit Price Rs Total Amount RsArun GuptaNo ratings yet
- Motor Current RM TechnicalDocument1 pageMotor Current RM TechnicalPhani VarmaNo ratings yet
- Specifications Water Supply PumpsDocument14 pagesSpecifications Water Supply PumpsArun GuptaNo ratings yet
- 34 ST 25 35Document112 pages34 ST 25 35Arun GuptaNo ratings yet
- Filter DataDocument10 pagesFilter DataArun GuptaNo ratings yet
- Sugar Storage in SilosDocument5 pagesSugar Storage in SilosArun GuptaNo ratings yet
- Sulphur BurnerDocument1 pageSulphur BurnerArun GuptaNo ratings yet
- Vibration Unit Converter R 1Document4 pagesVibration Unit Converter R 1Arun GuptaNo ratings yet
- Instrument Annexure IDocument4 pagesInstrument Annexure IArun GuptaNo ratings yet
- ScreensDocument6 pagesScreensArun GuptaNo ratings yet
- About NPCS PDFDocument24 pagesAbout NPCS PDFArun GuptaNo ratings yet
- Voltage Drop FormulasDocument6 pagesVoltage Drop FormulasArun GuptaNo ratings yet
- Digital Online TDS MeterDocument6 pagesDigital Online TDS MeterArun GuptaNo ratings yet
- Dehumid Prod ListDocument9 pagesDehumid Prod ListArun GuptaNo ratings yet
- 3 TB - Dehumidification and The Psychrometric ChartDocument4 pages3 TB - Dehumidification and The Psychrometric Chartashfaq-matte-7993100% (2)
- Boiler AutomationDocument2 pagesBoiler AutomationkridhakenyaNo ratings yet
- Refrigerant Dehumidification TechnologyDocument4 pagesRefrigerant Dehumidification TechnologyUmarraj SaberwalNo ratings yet
- Vacuum DatasheetDocument5 pagesVacuum DatasheetArun GuptaNo ratings yet
- Sea WaterDocument39 pagesSea WaterArun GuptaNo ratings yet
- DonnellyDocument2 pagesDonnellyArun GuptaNo ratings yet
- 651Document18 pages651Arun GuptaNo ratings yet
- Sugar NamesDocument1 pageSugar NamesjradNo ratings yet
- Static Mixer DataDocument6 pagesStatic Mixer DataArun GuptaNo ratings yet
- JVK Filter Elements enDocument16 pagesJVK Filter Elements enArun GuptaNo ratings yet
- Install, Operate, Maintain Co-Gen Plant at Palwal Sugar MillDocument32 pagesInstall, Operate, Maintain Co-Gen Plant at Palwal Sugar MillArun GuptaNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- BIORANSFORMATIONDocument79 pagesBIORANSFORMATIONBandameedi RamuNo ratings yet
- Natural Hydrating Milk CleanserDocument1 pageNatural Hydrating Milk CleanserNadaNursetiyantiNo ratings yet
- Cambridge IGCSE: Chemistry 0620/21Document16 pagesCambridge IGCSE: Chemistry 0620/21Mina AbdouNo ratings yet
- Matter Is The Physical Material of The UniverseDocument10 pagesMatter Is The Physical Material of The UniversePrince QureshiNo ratings yet
- Tablas de AlcoholesDocument13 pagesTablas de AlcoholesHugo miranda86% (7)
- The Accurate Measurement of Contact Angle, Phase ContactDocument8 pagesThe Accurate Measurement of Contact Angle, Phase ContactaleiviNo ratings yet
- Chemistry Lab Seating PlanDocument5 pagesChemistry Lab Seating PlanPranav EdaraNo ratings yet
- VW 50133 en 2021-06Document15 pagesVW 50133 en 2021-06xu zhangNo ratings yet
- Laboratory Report Sheet 02 Properties of SolidsDocument10 pagesLaboratory Report Sheet 02 Properties of SolidsCzarina RelleveNo ratings yet
- Practical Wet Test Acid Radical-1Document5 pagesPractical Wet Test Acid Radical-1psyxs4tsv9No ratings yet
- Assignment 1Document3 pagesAssignment 1Yeleti FamilyNo ratings yet
- Affect of Cooking On Quality of PulsesDocument14 pagesAffect of Cooking On Quality of PulsesBotany Department100% (1)
- Surface Vehicle Standard: Rev. MAY97Document4 pagesSurface Vehicle Standard: Rev. MAY97anupthattaNo ratings yet
- Donald L Smith Gamma PatentDocument34 pagesDonald L Smith Gamma PatentjradNo ratings yet
- Estimating Crystallite Size Using XRDDocument105 pagesEstimating Crystallite Size Using XRDKrizelle Ann HugoNo ratings yet
- Form 1 PhysicsDocument75 pagesForm 1 PhysicsClinton ChikengezhaNo ratings yet
- Sequential Extraction ProcedureDocument1 pageSequential Extraction ProcedureGangi Reddy UbbaraNo ratings yet
- Heterogeneous CatalystDocument24 pagesHeterogeneous Catalystlalukalu420No ratings yet
- New Microsoft Office Word DocumentDocument26 pagesNew Microsoft Office Word DocumentAnitha SathishNo ratings yet
- Technical Documentation Sika AnchorFix-1!03!2012Document19 pagesTechnical Documentation Sika AnchorFix-1!03!2012Anonymous Vi1lrHNo ratings yet
- AF3 Summary of Graduation ThesisDocument1 pageAF3 Summary of Graduation ThesisvvvNo ratings yet
- Drinking Water Treatment Design CalculationsDocument13 pagesDrinking Water Treatment Design Calculationssalil dubey100% (1)
- Development and Validation of Bioanalytical Methods For Quantitative Analysis of Gefitinib by Using Uv-Visible SpectrophotometryDocument13 pagesDevelopment and Validation of Bioanalytical Methods For Quantitative Analysis of Gefitinib by Using Uv-Visible SpectrophotometryPamarthi TejaswiNo ratings yet
- Org. Chem. (Chapter 8)Document25 pagesOrg. Chem. (Chapter 8)Jia LinNo ratings yet
- Development of Yondelis (Trabectedin, ET-743) - A Semisynthetic Process Solves The Supply ProblemDocument16 pagesDevelopment of Yondelis (Trabectedin, ET-743) - A Semisynthetic Process Solves The Supply ProbleminsaNo ratings yet
- Science X QP Set BDocument7 pagesScience X QP Set BYogesh KhannaNo ratings yet
- Effect of The Use of Ceramic Filters in Steel CastingDocument6 pagesEffect of The Use of Ceramic Filters in Steel CastingJavier Escalante VillanuevaNo ratings yet
- Chapter 8Document6 pagesChapter 8Jerico LlovidoNo ratings yet
- Excimer Lasers: Edited by Ch. K. RhodesDocument275 pagesExcimer Lasers: Edited by Ch. K. RhodesSujay SwainNo ratings yet
- Ptable 6Document1 pagePtable 6RawandNo ratings yet