Professional Documents
Culture Documents
Unit 8 Test
Uploaded by
mamazookeeprOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Unit 8 Test
Uploaded by
mamazookeeprCopyright:
Available Formats
Name: _______________________________ Date: _________________ Period: ______________
Unit 8 Test
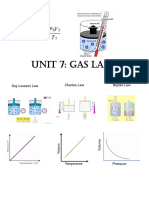
Fill in the blank. Write increases or decreases to complete each relationship. (1 point each) 1. 2. 3. 4. 5. As volume increases, pressure ________________________. As volume increases, temperature _______________________. As pressure increases, temperature ______________________. As pressure increases, concentration _____________________. As force decreases, pressure ______________________.
True or False. Write true or false on the line. (1 point each) 6. __________ The Kinetic Theory of Matter deals with moving particles. 7. __________ Gases can be collected by trapping them over water. 8. __________ Real gases exist in the world around us. 9. __________ Densities of gases are lower than 1 g/mL. 10. __________ If the barometric pressure is falling, that means good weather is coming. 11. __________ The troposphere contains the most air particles. 12. __________ Temperature is a measure of how fast particles are moving. Multiple Choice. Circle the correct response. (1 point each) 13. The gas that makes up the highest percentage of the atmosphere is: a. Nitrogen b. Oxygen c. Water vapor d. argon 14. Atmospheric pressure is measured using a: a. Thermometer b. Hygrometer c. Barometer 15. Squeezing gas particles together is known as: a. Expansion b. Effusion c. Compressibility
d. Speedometer
d. Volume
16. When particles collide with the sides of a container, they create: a. Volume b. Pressure c. Diffusion d. Temperature 17. The spreading of a gas through a small opening is: a. Diffusion b. Osmosis c. Fluidity d. Effusion 18. The number of gas particles present is called the: a. Concentration b. Volume c. Temperature
d. Pressure
Gas Law Problems. Write the answer with the correct unit. 19. A gas has a beginning pressure of 2.95 atm and a volume of 5.15 L. If the ending pressure is 4.23 atm, what is the ending volume?
20. A gas has a beginning temperature of 300 K and a volume of 4.06 L. If the ending temperature is 176 K, what is the ending volume?
21. A gas has a beginning pressure of 2.09 atm, a temperature of 268 K, and a volume of 0.450 L. If the ending pressure is 1.2 atm and the temperature is 149 K, what is the ending volume?
22. A system of gases has a total pressure of 760 mm. The pressures of two of the gases in the system are 233.5 mm and 138.67 mm. What is the pressure of the remaining gas?
23. Hydrogen was collected over water. The total pressure of the system was 730 mm. The water vapor pressure was 45.2 mm. What was the pressure of the hydrogen gas?
24. A gas has a pressure of 1.94 atm, a temperature of 330 K, and 0.74 moles. What is the volume of the gas?
25. 2 Al + 6 HCl 3 H2 + 2 AlCl3 a. 6.8 mol of Al are used. How many liters of H2 are produced?
b. 0.17 L of H2 are produced. How many grams of Al are used?
You might also like
- Physical and Chemical Equilibrium for Chemical EngineersFrom EverandPhysical and Chemical Equilibrium for Chemical EngineersRating: 5 out of 5 stars5/5 (1)
- Unit 8 Review AnswersDocument2 pagesUnit 8 Review AnswersmamazookeeprNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- Pre-Assessment Directions: Answer The Following Questions Below About Volume-Pressure Relationship and Write Your Answer inDocument2 pagesPre-Assessment Directions: Answer The Following Questions Below About Volume-Pressure Relationship and Write Your Answer inMa'am MercadoNo ratings yet
- Gas Laws ExplainedDocument6 pagesGas Laws ExplainedRicki HanNo ratings yet
- Unit Review Part 2 2022Document3 pagesUnit Review Part 2 2022tjqxqpxzx5No ratings yet
- Boyle's Law Computer SimulationDocument3 pagesBoyle's Law Computer SimulationAnonymous 52Z8ZFkv0% (1)
- Study Guide Gases StudentDocument6 pagesStudy Guide Gases StudentnicoNo ratings yet
- Science 10Document3 pagesScience 10Anna Glodove SeredricaNo ratings yet
- Pressure Review and PracticeDocument2 pagesPressure Review and PracticeVixenNo ratings yet
- Science 10 - Week 28Document4 pagesScience 10 - Week 28Mira VeranoNo ratings yet
- Understanding GasesDocument30 pagesUnderstanding GasesMohammad Amjad KhanNo ratings yet
- Gay LussacDocument4 pagesGay LussacdickaardianNo ratings yet
- Gas Laws LabDocument4 pagesGas Laws LabBrady DeNioNo ratings yet
- Gas Laws Test ReviewDocument2 pagesGas Laws Test ReviewXzyle1213No ratings yet
- Chapter-13 Assessment Gases Student EditableDocument5 pagesChapter-13 Assessment Gases Student EditableFabricio FernandezNo ratings yet
- Unit 8 Notes: Kinetic Theory of MatterDocument5 pagesUnit 8 Notes: Kinetic Theory of MattermamazookeeprNo ratings yet
- Grade 10 Fourth Quarter ExamDocument2 pagesGrade 10 Fourth Quarter Examnina lykka calaraNo ratings yet
- Merce T Boiler ExperimentDocument14 pagesMerce T Boiler ExperimentjevaughnNo ratings yet
- LEARNING ACTIVITY SHEET 3 (Behavior of Gases) - Science 10 (4th Quarter)Document1 pageLEARNING ACTIVITY SHEET 3 (Behavior of Gases) - Science 10 (4th Quarter)cherrymaeregalario2001No ratings yet
- Gizmo - Conservation of Energy in A SystemDocument5 pagesGizmo - Conservation of Energy in A SystemJustin Wen33% (3)
- Eastern Visayas State University: Education DepartmentDocument5 pagesEastern Visayas State University: Education DepartmentMichelle EscalienteNo ratings yet
- Topic 7 Practice PacketDocument46 pagesTopic 7 Practice Packetsg 85No ratings yet
- Eastern Visayas State University-Ormoc City CampusDocument3 pagesEastern Visayas State University-Ormoc City Campuskhellian villameroNo ratings yet
- Orca Share Media1644931117177 6899341164500579200Document6 pagesOrca Share Media1644931117177 6899341164500579200Lae RamirezNo ratings yet
- Chem QuestionsDocument2 pagesChem QuestionsJullia Lyn Marie FuentesNo ratings yet
- Gas Laws Practice WorksheetDocument2 pagesGas Laws Practice WorksheetEmily VennenNo ratings yet
- Chemistry (Practice Test) Name - Chapter 12 (The Gas Laws)Document4 pagesChemistry (Practice Test) Name - Chapter 12 (The Gas Laws)Cenando BodanioNo ratings yet
- AP Chemistry Unit 10 Packet 1 AnswersDocument26 pagesAP Chemistry Unit 10 Packet 1 AnswersBrandon BaxterNo ratings yet
- Practical 3: Gas LawsDocument2 pagesPractical 3: Gas LawsSandy NgNo ratings yet
- Lab 8 - Specific Heat Capacity (Elctrical Method)Document4 pagesLab 8 - Specific Heat Capacity (Elctrical Method)Shazira AllyNo ratings yet
- Ch. 10 Extra Review WSDocument4 pagesCh. 10 Extra Review WSPrecious BucayNo ratings yet
- Module 3 Activities G8Document9 pagesModule 3 Activities G8Julia Geonzon LabajoNo ratings yet
- Activity-5-Science-10-4th-QDocument2 pagesActivity-5-Science-10-4th-QStarskie MonacarNo ratings yet
- 396 Usando La Energía para Observar Cambios QuímicosDocument2 pages396 Usando La Energía para Observar Cambios QuímicosBRYAN ARNOLDO AGUILAR GOMEZNo ratings yet
- Labsheet 1Document5 pagesLabsheet 1raidahNo ratings yet
- 18.2.21 1.03.21 Reactions 2 Working From Home Booklet PART 2 AnswersDocument33 pages18.2.21 1.03.21 Reactions 2 Working From Home Booklet PART 2 Answerslokapavani_senthilNo ratings yet
- Gay-Lussac's Law experimentDocument1 pageGay-Lussac's Law experimentValen MonterozoNo ratings yet
- Understanding Gas Laws with Kinetic Molecular Theory SimulationsDocument5 pagesUnderstanding Gas Laws with Kinetic Molecular Theory SimulationsAdam CrabbNo ratings yet
- Water Vapor Capacity of AirDocument3 pagesWater Vapor Capacity of AirAhmed EldalyNo ratings yet
- Science 10 - Week 27Document3 pagesScience 10 - Week 27Mira VeranoNo ratings yet
- Additivity of Heats of Reaction: Hess's LawDocument5 pagesAdditivity of Heats of Reaction: Hess's Lawnipuna920% (1)
- Physical Chemistry Mid Term ExamDocument4 pagesPhysical Chemistry Mid Term ExamMaricar DimasNo ratings yet
- Introduction to Gas Laws SimulationDocument5 pagesIntroduction to Gas Laws SimulationMika VaughnNo ratings yet
- Gas Laws LabDocument6 pagesGas Laws LabzhuzaiNo ratings yet
- Chemistry 5 NotesDocument67 pagesChemistry 5 Notesdbessen2No ratings yet
- Module 1 - GasesDocument13 pagesModule 1 - GasesRuth AquinoNo ratings yet
- Exp 15 Molecular Weight Determination of Vapor PDFDocument7 pagesExp 15 Molecular Weight Determination of Vapor PDFLisette Joyce LolaNo ratings yet
- Labsheet 4Document6 pagesLabsheet 4raidahNo ratings yet
- CH 12 Study GuideDocument8 pagesCH 12 Study GuideyawahabNo ratings yet
- Gas Laws Exam: Escribe Tu Nombre CompletoDocument4 pagesGas Laws Exam: Escribe Tu Nombre CompletoEver MendezNo ratings yet
- Boyle's and Charles's Laws WorksheetDocument3 pagesBoyle's and Charles's Laws WorksheetARVIN CONCHANo ratings yet
- Molar Mass of Metal PV NRT LabDocument6 pagesMolar Mass of Metal PV NRT LabYi LingNo ratings yet
- Chapter 5 - Gases Properties of Gases: Atmospheric Pressure or Barometric Pressure?Document15 pagesChapter 5 - Gases Properties of Gases: Atmospheric Pressure or Barometric Pressure?Hera EstoseNo ratings yet
- charles-lawDocument3 pagescharles-lawsarausos.zoe08No ratings yet
- QuizDocument2 pagesQuizAkatew Haile MebrahtuNo ratings yet
- Labsheet 3Document5 pagesLabsheet 3raidahNo ratings yet
- 2.1.3-Ans-Gases & Amp Absolute Scale of TempDocument10 pages2.1.3-Ans-Gases & Amp Absolute Scale of TempYara AlaaNo ratings yet
- Week 4Document42 pagesWeek 4Tonepher CaballeroNo ratings yet
- Elements of Modern Style QuestionsDocument2 pagesElements of Modern Style QuestionsmamazookeeprNo ratings yet
- Chemical Formula QuizDocument3 pagesChemical Formula QuizmamazookeeprNo ratings yet
- Formula Sheet 2Document1 pageFormula Sheet 2mamazookeeprNo ratings yet
- Hydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andDocument1 pageHydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andmamazookeeprNo ratings yet
- Formula Sheet 1Document1 pageFormula Sheet 1mamazookeeprNo ratings yet
- Build A Trend LabDocument2 pagesBuild A Trend LabmamazookeeprNo ratings yet
- Elements of Modern Style ArticleDocument5 pagesElements of Modern Style ArticlemamazookeeprNo ratings yet
- Trends ReviewDocument2 pagesTrends ReviewmamazookeeprNo ratings yet
- Practice Symbol Quiz AnswersDocument1 pagePractice Symbol Quiz AnswersmamazookeeprNo ratings yet
- Electron Configuration SheetDocument2 pagesElectron Configuration SheetmamazookeeprNo ratings yet
- Practice Symbol QuizDocument1 pagePractice Symbol QuizmamazookeeprNo ratings yet
- Shorthand ConfigurationsDocument1 pageShorthand ConfigurationsmamazookeeprNo ratings yet
- Lab - Which Side Are You OnDocument3 pagesLab - Which Side Are You OnmamazookeeprNo ratings yet
- Periodic+Table XDocument38 pagesPeriodic+Table XmamazookeeprNo ratings yet
- Unit 4 ReviewDocument2 pagesUnit 4 ReviewmamazookeeprNo ratings yet
- Atoms and The PTDocument16 pagesAtoms and The PTmamazookeeprNo ratings yet
- Unit 4 Test 2009Document2 pagesUnit 4 Test 2009mamazookeeprNo ratings yet
- Unit 2 Re TestDocument2 pagesUnit 2 Re TestmamazookeeprNo ratings yet
- Answers To Example Problems From Class Regular ConfigurationsDocument1 pageAnswers To Example Problems From Class Regular ConfigurationsmamazookeeprNo ratings yet
- Unit 3 Practice TestDocument3 pagesUnit 3 Practice TestmamazookeeprNo ratings yet
- Light Equation SheetDocument1 pageLight Equation SheetmamazookeeprNo ratings yet
- Rainbow of ColorsDocument2 pagesRainbow of ColorsmamazookeeprNo ratings yet
- Subatomic Particle WorksheetDocument1 pageSubatomic Particle WorksheetmamazookeeprNo ratings yet
- Mole SheetDocument1 pageMole SheetmamazookeeprNo ratings yet
- Atomic History Old XDocument32 pagesAtomic History Old XmamazookeeprNo ratings yet
- Unit Analysis ProblemsDocument1 pageUnit Analysis ProblemsmamazookeeprNo ratings yet
- Average Weighted Atomic MassDocument2 pagesAverage Weighted Atomic MassmamazookeeprNo ratings yet
- Lab Report Format: Title Source Name Partner Date PurposeDocument2 pagesLab Report Format: Title Source Name Partner Date PurposemamazookeeprNo ratings yet
- Popcorn LabDocument1 pagePopcorn LabmamazookeeprNo ratings yet
- Sample Lab ReportDocument3 pagesSample Lab Reportmamazookeepr63% (8)
- 5.plumbing - FixturesDocument33 pages5.plumbing - FixturesMenshafiNo ratings yet
- Tests for Organic MoleculesDocument7 pagesTests for Organic MoleculesICAMisterPNo ratings yet
- Newtons Laws With Good PicturesDocument30 pagesNewtons Laws With Good PicturesMohammed RiyazuddinNo ratings yet
- Hilts 105Document277 pagesHilts 105Wesley CheungNo ratings yet
- Ohmic Heating Process ExplainedDocument37 pagesOhmic Heating Process ExplainedKaran Jethva100% (1)
- Substance Chemistry Lesson 2Document16 pagesSubstance Chemistry Lesson 2samsonNo ratings yet
- Concrete Aggregates: Standard Specification ForDocument15 pagesConcrete Aggregates: Standard Specification ForHasanalmahmudNo ratings yet
- Lecture Notes For CO3 (Part 1) : Forced and Free Convection Heat TransferDocument43 pagesLecture Notes For CO3 (Part 1) : Forced and Free Convection Heat TransferSarindran RamayesNo ratings yet
- Physical Pharmacy Lab - Post LabsDocument90 pagesPhysical Pharmacy Lab - Post LabsFlorence Lynn BaisacNo ratings yet
- Lehninger Biochem Ch 1-2 ReviewDocument13 pagesLehninger Biochem Ch 1-2 ReviewHugo DuarteNo ratings yet
- International Chemistry Olympiad Problems Volume 01 (1968-1988)Document408 pagesInternational Chemistry Olympiad Problems Volume 01 (1968-1988)Science Olympiad Blog100% (5)
- AIChE Pocket HandbookDocument64 pagesAIChE Pocket HandbookDinesh KanaujiyaNo ratings yet
- Wondraczek Et Al. - 2022 - Advancing The Mechanical Performance of Glasses PDocument25 pagesWondraczek Et Al. - 2022 - Advancing The Mechanical Performance of Glasses PAlireza BagherpourNo ratings yet
- How Enzymes Work - The Role of Proteins in Chemical ReactionsDocument12 pagesHow Enzymes Work - The Role of Proteins in Chemical ReactionsRahil BhavanNo ratings yet
- 31.2-General Wave Popeties-Cie Igcse Physics Ext-Theory-QpDocument12 pages31.2-General Wave Popeties-Cie Igcse Physics Ext-Theory-Qpnityam bajajNo ratings yet
- Bpharmacy 1 Sem Pharmaceutics 1 Set P 2018Document3 pagesBpharmacy 1 Sem Pharmaceutics 1 Set P 2018Mandlik PritiNo ratings yet
- SPE 55633 ASP Cambridge FieldDocument6 pagesSPE 55633 ASP Cambridge FieldSuper YaniNo ratings yet
- Aim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemDocument3 pagesAim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemSachin SoniNo ratings yet
- 1 What - Are - Enzymes PDFDocument16 pages1 What - Are - Enzymes PDFtmlNo ratings yet
- Pericyclic ReactionsDocument5 pagesPericyclic ReactionsNurul HidayahNo ratings yet
- Sika Rep Fine MSDocument4 pagesSika Rep Fine MSmohghareib80No ratings yet
- The Otto Schmidt's Inter-Stellar Dust TheoremDocument1 pageThe Otto Schmidt's Inter-Stellar Dust TheoremKC CampilanNo ratings yet
- Co(III) Coordination Compounds Synthesis ReactionsDocument28 pagesCo(III) Coordination Compounds Synthesis ReactionsRahul GogiaNo ratings yet
- Exp5 The Green Minded 3735Document16 pagesExp5 The Green Minded 3735CyberR.DomingoNo ratings yet
- Anlage C DQC III-18 La DQC IV-18 LaDocument5 pagesAnlage C DQC III-18 La DQC IV-18 LaPEDRONo ratings yet
- Jan 23 WCH12 SolvedDocument28 pagesJan 23 WCH12 Solvedthe dsNo ratings yet
- 3c Zeroth Law of ThermodynamicsDocument5 pages3c Zeroth Law of ThermodynamicsSamuel BoujeeNo ratings yet
- AP Physics 1 Free ResponseDocument2 pagesAP Physics 1 Free ResponseafhNo ratings yet
- Basf Masterglenium 118 TdsDocument2 pagesBasf Masterglenium 118 TdsFatma IbrahimNo ratings yet
- PH Ysicsguide: Basic Concepts of Statistical MechanicsDocument14 pagesPH Ysicsguide: Basic Concepts of Statistical MechanicsMNo ratings yet
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceFrom EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceRating: 4.5 out of 5 stars4.5/5 (23)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- Quantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishFrom EverandQuantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishRating: 4.5 out of 5 stars4.5/5 (18)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismFrom EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismRating: 4 out of 5 stars4/5 (500)
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- Lost in Math: How Beauty Leads Physics AstrayFrom EverandLost in Math: How Beauty Leads Physics AstrayRating: 4.5 out of 5 stars4.5/5 (125)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterFrom EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterRating: 4.5 out of 5 stars4.5/5 (409)
- Strange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsFrom EverandStrange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsRating: 4 out of 5 stars4/5 (94)
- Quantum Physics: What Everyone Needs to KnowFrom EverandQuantum Physics: What Everyone Needs to KnowRating: 4.5 out of 5 stars4.5/5 (48)
- Paradox: The Nine Greatest Enigmas in PhysicsFrom EverandParadox: The Nine Greatest Enigmas in PhysicsRating: 4 out of 5 stars4/5 (57)
- Bedeviled: A Shadow History of Demons in ScienceFrom EverandBedeviled: A Shadow History of Demons in ScienceRating: 5 out of 5 stars5/5 (5)
- Too Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldFrom EverandToo Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldRating: 4.5 out of 5 stars4.5/5 (8)
- The End of Everything: (Astrophysically Speaking)From EverandThe End of Everything: (Astrophysically Speaking)Rating: 4.5 out of 5 stars4.5/5 (155)
- The Holographic Universe: The Revolutionary Theory of RealityFrom EverandThe Holographic Universe: The Revolutionary Theory of RealityRating: 4.5 out of 5 stars4.5/5 (76)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeFrom EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNo ratings yet
- In Search of Schrödinger’s Cat: Quantum Physics and RealityFrom EverandIn Search of Schrödinger’s Cat: Quantum Physics and RealityRating: 4 out of 5 stars4/5 (380)
- Black Holes: The Key to Understanding the UniverseFrom EverandBlack Holes: The Key to Understanding the UniverseRating: 4.5 out of 5 stars4.5/5 (13)
- Starry Messenger: Cosmic Perspectives on CivilizationFrom EverandStarry Messenger: Cosmic Perspectives on CivilizationRating: 4.5 out of 5 stars4.5/5 (158)
- The Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsFrom EverandThe Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsRating: 5 out of 5 stars5/5 (5)