Professional Documents
Culture Documents
Suga
Uploaded by
Sean HuffCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Suga
Uploaded by
Sean HuffCopyright:
Available Formats
J. Food Chem. Nutr. 01 (02) 2013.
78-83
Available Online at ESci Journals
Journal of Food Chemistry and Nutrition
ISSN: 2307-4124 (Online), 2308-7943 (Print)
http://www.escijournals.net/JFCN
PHYSICOCHEMICAL PROPERTIES OF OIL EXTRACTED FROM THE HOT AND COLD EXTRACTED RED PITAYA (HYLOCEREUS POLYRHIZUS) SEEDS
Murugesu, aAbdul A. Ariffin*, aTan C. Ping, bBoo H. Chern of Food Technology, Faculty of Food Science and Technology, University Putra Malaysia. b Department of Food Service and Management, Faculty of Food Science and Technology, University Putra Malaysia.
a Department aSuganya
ABSTRACT
The red pitaya, Hylocereus Polyrhizus, well-known for its tasty pulp attached with sticky and mucilage coated seeds. The seed oils properties are highly influenced by the seed extraction techniques. Therefore, effects of two different seed extraction techniques on some quality characteristics of pitaya seed oil of the red cultivar, Hylocereus Polyrhizus were investigated. The techniques are referred as the hot and cold method applied in extracting clear mucilage free red pitaya seeds. The oil obtained from the seeds were analysed for its free fatty acids, peroxide, p-anisidine and the totox values. The fatty acids and triacylglycerols separated using analytical chromatographic technique. The free fatty acids value and the oxidation products measure of both methods showed no significant differences. The GC analysis for the hot extracted seed oil showed the presence of palmitic (15.712%), oleic (24.889%) and linoleic acid (48.699%) while the cold extracted seed oil exhibited slightly higher content of palmitic (15.778%), oleic (23.566%) and linoleic acid (49.807%). The HPLC analysis triacylglycerols of the pitaya seed oil showed Linoleic: Linoleic: Linoleic (LLL) and Oleic: Linoleic: Linoleic (OLL) dominated the TAG composition of the oil with the average percentage of 14.065% and 13.990% for the cold extracted seed oil and 15.620% and 15.795% for the hot one. The result shows that different seed extraction techniques influence the oil yield and the seed oils properties. Keywords: Pitaya seed oil, Hylocereus polyrhizus, Essential fatty acids, Linoleic acid, Triacylglycerols. INTRODUCTION Pitaya or better known as dragon fruit has become one of the most desirable fruit in the market in recent times due to its sweet taste and texture (Lim et al., 2010). The red pitaya, Hylocereus polyrhizus (Hylocereus polyrhizus) especially has become the most attracted pitaya variety for its red-violet flesh and sweetness that signifies the high carbohydrate content in it (Wichienchot et al., 2010). The red pitaya also reported to contain high fiber, protein and minerals (Ruzainah et al., 2009). Another study done by Rebecca et al. (2010) has revealed the probiotic properties and high anti-oxidant level in red pitaya fruit. The red pitaya flesh impregnated with minute distinctive seeds which are coated with mucilage. This morphological condition leads to different seed extraction ideas that could serve on gaining clear bright * Corresponding Author: Email ID: abdulazis@putra.upm.edu.my 2013 ESci Journals Publishing. All rights reserved. seeds to analyse its potential contents. The principal reason behind this study is to attempt on 2 different seed extraction techniques (hot & cold method) in order to obtain clear pitaya seeds. The seed oil quality can be affected by the seed extraction method and condition especially for pitaya seeds due to its morphological condition. The challenge is extremely in the recovery of seeds from its gel-like coating (mucilage) that envelope every seed. The hot method applies heat hydrolysis of the mucilage with endogenous water. In contrast, the mucilage was hydrolyzed with acid at ambient temperature (25C) in the cold method (Nemati et al., 2010). Oil extracted from these group of seeds were analysed for its free fatty acids and oxidation products. Besides that, triacylglycerols and fatty acid composition in the oil were also determined. About 30% of oil yield of the red pitaya seed which is rich in essential fatty acids was reported by Ariffin et al. (2009). A complete diet includes a well-balanced intake of essential fatty acids (EFA) and the acids have been
78
J. Food Chem. Nutr. 01 (02) 2013. 78-83 reported to exhibit multiple nutraceutical roles in human well-being. Meharban Singh (2005) in his review stated that deficiency in fatty acid intake may lead to impaired development in brain cells, especially in infants. Triacylglycerols (TAGs) are the main constituent of vegetable oil and play an important role as transporters of fatty acids which eventually serve as an energy source. Complete hydrolysis of TAG gives glycerol and fatty acids. In plant seeds oil, the breakdown of TAGs will eventually increase the free fatty acids (FFA) level in the oil thus affecting the oil quality. FFA value is one of the important parameter to evaluate the oil quality as well (Nyam et al., 2009). All the quality analyses relate to the effect of seed extraction techniques that are being implemented in this study. This study is aimed to compare the physicochemical properties of the oil obtained from the respective hot and cold extraction processes. MATERIALS AND METHODS Materials: Mature red pitaya fruits, H. polyrhizus1 were purchased from the local farm located at Sepang, Selangor, Malaysia. All the chemicals used were of analytical grade purchased from Merck (Darmstadt, Germany). The fatty acid methyl ester (FAME) standard mixture was purchased from the Supelco Co. (United States of America). Methodology: Seed extraction techniques Hot method: The fruits purchased were each wrapped with aluminum foil and autoclaved for an hour. The hydrolyzed fruits were cooled and aluminum foil removed before the skins were carefully peeled. The hydrolyzed flesh recovered and mixed vigorously with water to allow complete separation of seeds from the flesh. The fine seeds that settled down at the bottom were collected and air dried (Ariffin et al., 2009). Cold method: The fruits were peeled and the flesh sliced into smaller size. The sliced fruit flesh transferred into the depulper to separate the seeds from the flesh. The seeds each enveloped with the mucilage were collected. The seeds were treated with hydrochloric acid; the mucilage free seeds were water-washed extensively. The hydrolyzed mucilage sieved and the seeds recovered and air dried (Vishwanath et al., 2006; Nemati et al., 2010). Oil extraction: 10 grams of dried seeds were finely ground and the oil extracted by solvent extraction using hexane (boiling point; 69 C). The solvent then evaporated using the rotary evaporator to obtain the oil (Besbes et al., 2005). Physicochemical properties: The physicochemical properties of the oil recovered were analysed using the routine AOCS method. The free fatty acids (FFA) were expressed as percentage of oleic acid based on the titration volume. The primary oxidation product, the peroxides value (PV) was expressed in milliequivalents of oxygen per kg of oil (mEq of O2/kg) while the secondary oxidation level was determined by measuring the p-anisidine value (p-AV). The acid value was calculated from the results obtained from the FFA. Totox value that indicates the total oxidation value (2PV + pAV) using the standard formula and the values obtained from the oxidation measurement tests. Fatty acid analysis using Gas chromatography: Pitaya oil (100mg) was esterified to fatty acid methyl ester using sodium methoxide and the different esterified fatty acid constituents were separated and determined using gas chromatography (Hewlett-Packard 6890 series) fitted with flame ionization detector using the column BPX-70 (60 m x 0.25 mm, internal diameter: 0.2 m) from Varian, Inc. with the oven condition of 115 C that raising to 180 C at the rate of 8 C/ minute and held for 10 minutes. It then allowed to reach up to 240 C at the rate of 8 C/ minute and held for 10 minutes (Ariffin et al., 2009). The fatty acids were identified by comparing the retention time of the sample peaks with the FAME3 standard mixture peaks and the result expressed in percentage of distribution (Ixtaina et al., 2011). Triglycerides: To prepare a 5% (w/v) of the test sample solution, accurately 50L of oil pipetted into a centrifuge tube (2mL) and dissolved with 950 L acetone and the tube sealed and vortex for a few seconds. The dissolved fat solution was filtered through the 0.45 m syringe filter directly into the HPLC vials. The clear sample solution (10 L) injected into the pre-optimized analytical conditions equipped with a refractive index detector, flow rate of 1.5 ml/min using Hichrom C18 column (15cm, 2.1mm i.d., 5m) as the stationary phase and mobile phase acetone: acetonitrile (50:50; v/v) mixture. Peak identification achieved by comparing the retention time of the sample peak with the alternative reference peak of soybean oil. Statistical analysis: The analyses were carried out in triplicates. The data analyzed using Minitab version 14.0
79
J. Food Chem. Nutr. 01 (02) 2013. 78-83 where analysis of variance (ANOVA) was applied to the data comprising FFA, AV, PV, p-AV and Totox measurement. Tukeys test at 5% significance applied to define on the data significant differences. RESULT AND DISCUSSION Physicochemical Properties: The physicochemical properties of the oil extracted from the hot and cold extracted seeds were analyzed and tabulated as below; Cold 0.108 0.01a 0.189 0.02a 0.124 0.01b 0.399 0.04a 0.398 0.04a
Table 1: Physicochemical properties of hot & cold extracted RP seed oil. Hot Physicochemical characteristic Free Fatty Acid, FFA (%) 0.119 0.02a Acid value (%) Peroxide value (mEq O2 / kg) p-Anisidine value Totox (2PV + p-AV) Based on the results displayed in table 1, there are no significant differences between the free fatty acids, acid and p-anisidine value of both the hot and cold method. The oil contains 0.119% and 0.108% of free fatty acids for hot and cold method respectively (Refer Table 1). The FFA value is considerably lower for any crude purified seed oil. This indicates the absence of enzymatic reaction (lipase) which would contribute to elevated FFA level in plant seed oil. Sometimes the FFA value of unrefined seed oil can go up to 3% depending upon their extraction method. The peroxide value is the index of rancidity, which is substantially lower for the red pitaya seed oil. This may be due to auto-oxidation that take place during the sample handling prior to analysis. The secondary stage of oxidation that occurs when the hydroperoxides decompose to form aldehydes was observed in this seed oil. However the anisidine value in this case is considerably lower in both methods based on the quality specifications for plant oils. 0.205 0.04a 0.105 0.01a 0.349 0.04a 0.441 0.04a
Results are expressed as mean SD for 3 replications, Alphabet within the same column were significantly difference p < 0.05.
Fatty acid composition: Fatty acids in the pitaya oil separated using gas chromatography and reported as percentage of distribution. The peaks were identified based on the retention time on the standard chromatogram composed of 19 components peak. The figures below show the fatty acid distribution in both hot and extracted seed oil. The chromatograms of the fatty acids of the H. polyrhizus1 hot and cold extracted H. polyrhizus seeds oil are shown in fig. 1 and fig.2. Both exhibit the similar range of fatty acid distribution. The linoleic acid content is the major fatty acid composition in the seed oil analyzed with the average percentage of 48% for the hot method and 49% of the cold one. These results are similar as reported by Ariffin et al. (2009) and Lim et al. (2012). There were no significant differences in the other fatty acid constituents of the hot and cold oil extracts with oleic at 24: 25% (hot: cold), palmitic 15:16% (hot: cold) and linolenic 1.3:1.2% (hot: cold).
Figure 1: Chromatogram of fatty acids of hot extracted seed oil.
80
J. Food Chem. Nutr. 01 (02) 2013. 78-83
Figure 2: Chromatogram of fatty acids of cold extracted seed oil. These values are comparable with other seed oil such as the grape seed oil which was reported to have about 60% of linoleic acids and the hempseed about 52% 62% of the essential omega- 6. The red pitaya seed oil contains higher palmitic acid compared to melon seed oil that contains only about 8.1% (De Mello et al., 2001). It can be emphasised here that high level of unsaturated fatty acids in our diet contributes in lowering of high blood cholesterol level (Baydar and Akkurt, 2001). Other fatty acids identified in the H. polyrhizus1 seed oil are the remaining long-chain fatty acid, erucic acid which presents between the ranges of 0.8% to 1.2%. Study done by Lim et al. (2010) showed the identification of erucic acid, calculated to be slightly lower compared to their report. The statistical analysis of the data showed that there are no significant differences of the palmitic, stearic and linoleic acid of both the hot and cold extracted seed oil. In contrast, the myristic, palmitoleic, oleic, cis-vaccenic and linolenic acids showed significant differences between the 2 techniques with the p value < 0.05. The numerical value of each fatty acid is presented in Table 2. Cold method 0.1717 0.00a 15.778 0.69a 0.841 0.00b 4.579 0.01a 23.566 0.23b 3.630 0.01b 49.087 0.41b 1.048 0.03b 1.196 0.04b
Table 2: Fatty acid composition of hot & cold extracted seed oil. Fatty acids Hot method Myristic (C14:0) 0.155 0.01a Palmitic (C16:0) 15.712 0.10a Palmitoleic (C16:1) 0.691 0.05a Stearic (C18:0) 4.832 0.16a Oleic (C18:1) 24.889 0.13a Cis- Vaccenic (C18:1a) 3.251 0.04a Linoleic (C18:2) 48.969 0.46a Linolenic (C18:3) 0.899 0.03a Erucic (C22:1) 0.908 0.04a Triacylglycerols: TAG was resolved by HPLC and was reported in relative percentage. Identification of the glycerides made based on the comparison to the reference chromatogram according to its elution carbon number (ECN) as well. Based on the relative percentage (%) tabulated below, the Oleic: Linoleic: Linoleic (OLL) and Linoleic: Linoleic: Linoleic (LLL) content are relatively higer than other TAGs combination. The OLL content in the purified crude pitaya seed oil is examined 81
Results are expressed as mean SD for 3 replications, Alphabet within the same column were significantly difference p < 0.05.
to be 15.620% & 15.795% while LLL, 14.065% and 13.990% for both the hot and cold extraction method respectively (Refer Table 3). Linoleic: Linolenic: Linolenic (LLnLn) was determined to be the least in oil analysed with the percentage of 0.805 and 0.695 for both the techniques respectively (Refer Table 3). This is comparable to other seed oil, for instant corn oil and sunflower oil was reported to have LLL of , 16.16% and 12.41% respectively (Andrikopoulos et al., 2001).
J. Food Chem. Nutr. 01 (02) 2013. 78-83
Figure 3: Chromatogram of RPSO. Table 3: Relative percentage of triacylglycerol present in RPSO. Triaclyglycerols Hot (Relative Percentage, %) LLnLn 0.805 0.08a LLLn 10.795 1.04a LLL 14.065 0.39a OOLn 12.065 0.0a PLLn 9.445 0.19 a OLL 15.620 0.26a PLL 3.475 0.05 a OOL 4.755 0.01a POL 10.875 0.22a PPL 3.410 0.08 a OOO 3.840 0.07 a SOL 2.720 0.07 a OOP 1.240 0.03 a PSL 1.520 0.01 a
Cold 0.6950 0.09a 11.515 0.02a 13.990 0.85a 12.155 0.01b 9.325 0.01a 15.795 0.11 a 3.570 0.03 a 4.490 0.03b 11.085 0.02a 3.485 0.09 a 3.800 0.04 a 2.785 0.01 a 1.260 0.03 a 1.575 0.05 a
P= Palmitic, S=Stearic, O=Oleic, L=Linoleic, Ln=Linolenic. Results are expressed as mean SD for 3 replications. Alphabet within the same column were significantly difference p < 0.05.
The statistical analysis of the TAG composition showed significant differences between the hot and cold for Oleic: Oleic: Linolenic (OOLn) and Oleic: Oleic: Linoleic (OOL) respectively while there are no significant differences among other TAG reported (Refer Table 3). CONCLUSION The study has shown that different seed extraction techniques may influence the seed oil composition and quality. Application of high temperature during the seed extraction does not affect the oil quality of the seed. The fatty acid composition of hot and cold extracted seed oil showed no significant differences. Moreover, higher seed yield was observed in hot procedure compared to the
cold method since seed separation using depulper contributed greater seed loss and was time consuming. The hot method is a one step process for the recovery of clean seeds. Analysis of other properties of the hot and cold extracted seed oil such as the anti-oxidant compounds may draw a better conclusion. ACKNOWLEDGEMENT The research work was supported by University Putra Malaysia fund; RUGS 1/2009 (Code: 03-918730011801). REFERENCES Andrikopoulos, N.K., I.G. Giannakis and V. Tzamtzis. 2001. Analysis of Olive Oil and Seed Oil
82
J. Food Chem. Nutr. 01 (02) 2013. 78-83 Triglycerides by Capillary Gas Chromatography as a Tool for the Detection of the Adulteration of Olive Oil. J. Chromatogr. Sci. 39: 137-145. Ariffin, A.A., J. Bakar, C.P. Tan, A.R. Rahman, R. Karim and C.C. Loi. 2009. Essential fatty acids of pitaya (dragon fruit) seed oil. Food Chem. 114: 561-564. Baydar, N.G. and M. Akkurt. 2001. Oil Content And Oil Quality Properties Of Some Grape Seeds. Turk J Agric For. 25: 163-168. Besbes, S., C. Blecker, C. Deroanne, G. Lognay, N.E. Drira and H. Attia. 2005. Heating effects on some quality characteristics of date seed oil. Food Chem. 91: 469-476. Ixtaina, V.Y., L.M. Marcela, V. Spotorno, C.M. Mateo, D.M. Maestri, B.W.K. Diehl, S.M. Nolasco, and M.C. Tomas. 2011. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Comp. Anal. 24: 166-174. Nyam, K.L., C.P. Tan, O.M. Lai, K. Long and Y.B. Che Man. 2009. Physicochemical properties and bioactive compounds of selected seed oils. LWT- Food Sci Technol. 42: 1396-1403. Lim, H.K., C.P. Tan, J. Bakar and S.P. Ng. 2012. Effects of Different Wall Materials on the Physicochemical Properties and Oxidative Stability of Spray-Dried Microencapsulated Red-Fleshed Pitaya (Hylocereus polyrhizus) Seed Oil. Food Bioprocess Tech. 5: 1220-1227. Lim, H.K., C.P. Tan, R. Karim, A.A. Ariffin and J. Bakar. 2010. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus Polyrhizus. Food Chem. 119: 1326-1331. De Mello, M.L.S., S.B. Pushkar and N. Narendra. 2001. Fatty and Amino acids composition of Melon (Cucumis melo Var. saccharinus) seeds. J. Food Comp. Anal. 14: 69-74. Meharban Singh. 2005. Essential fatty acids, DHA and Human Brain; Child Care and Dental Health Care, Noida, UP, India. Indian Journal of Pediatrics. 72 (3): 239-242. Rebecca, O.P.S., A.N. Boyce and S. Chandran. 2010. Pigment identification and antioxidant properties of red dragon fruit (Hylocereus Polyrhizus). Afr. J. Biotechnol. 9: 1450-1454. Nemati, H, Nazdar T, Azizi M, Arouiee H. 2010. The effect of seed extraction methods on seed quality of two cultivar's tomato (Solanum lycopersicum L.). Pak J Biol Sci. 13(17): 814-20. Ruzainah, A.J., A. Ridhwan, N.Z.C. Mahmod and R. Vasudevan. 2009. Proximate Analysis of Dragon Fruit (Hylocereus polyhizus). Am J Appl Sci. 6: 1341-1346. Wichienchot, S., M. Jatupornpipat and R.A. Rastall. 2010. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 120: 850 857. Vishwanath, K., B.S. Rajashekhar, V.P. Kalappa, V. Muniyappa and N. Adivayppar. 2006. Influence of seed extraction methods on seed quality in leaf curl resistant tomato varieties. J Asian Hortic. 2: 250-254.
83
You might also like
- Aaaaaaaaa Aaaaaaaaa Aaaaaaaaa Aaaaaaaaa Aaaaaaaaa AaaaaaaaaDocument4 pagesAaaaaaaaa Aaaaaaaaa Aaaaaaaaa Aaaaaaaaa Aaaaaaaaa AaaaaaaaaSean HuffNo ratings yet
- Asssssssss Ssssssssss Saaaaaaaa Aaaaaaaaa Aaaaaaaaa AaaaaaaaaDocument4 pagesAsssssssss Ssssssssss Saaaaaaaa Aaaaaaaaa Aaaaaaaaa AaaaaaaaaSean HuffNo ratings yet
- Business PlanDocument18 pagesBusiness PlanSean HuffNo ratings yet
- CCCCCCCCC CCCCCCCCC CCCCCCCCC CVVVVVVVV VVVVVVVVV VVVVVVVVVDocument2 pagesCCCCCCCCC CCCCCCCCC CCCCCCCCC CVVVVVVVV VVVVVVVVV VVVVVVVVVSean HuffNo ratings yet
- Dmi6601nutrition Panels v2Document4 pagesDmi6601nutrition Panels v2Sean HuffNo ratings yet
- Top Malaysian Universities Junior Marketing Role Status UpdateDocument1 pageTop Malaysian Universities Junior Marketing Role Status UpdateSean HuffNo ratings yet
- Applications of Palm Oil and Palm Kernel Oils in Different Food Products of BangladeshDocument6 pagesApplications of Palm Oil and Palm Kernel Oils in Different Food Products of BangladeshSean HuffNo ratings yet
- Chocolate ProductsDocument5 pagesChocolate ProductsaromazvwNo ratings yet
- Short guide to fill spacesDocument3 pagesShort guide to fill spacesSean HuffNo ratings yet
- XXCVNDocument3 pagesXXCVNSean HuffNo ratings yet
- VVVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVNNNDocument4 pagesVVVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVVVV VVVVVVVNNNSean HuffNo ratings yet
- Short document about lettersDocument2 pagesShort document about lettersSean HuffNo ratings yet
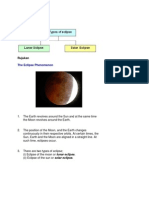
- Rujukan: The Eclipse PhenomenonDocument6 pagesRujukan: The Eclipse PhenomenonSean HuffNo ratings yet
- Ssssssssssssssss Ssssssssssssssss SSSSGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GgggggggssssssDocument4 pagesSsssssssssssssss Ssssssssssssssss SSSSGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GgggggggssssssSean HuffNo ratings yet
- KKKKKKKKKLLL LLLLLLLLLLLKKK KKKKKKKKKKK KKKKKKKKKKKL LLLLLLLLLLLLLLL LLLLLLLLLKKKKDocument2 pagesKKKKKKKKKLLL LLLLLLLLLLLKKK KKKKKKKKKKK KKKKKKKKKKKL LLLLLLLLLLLLLLL LLLLLLLLLKKKKSean HuffNo ratings yet
- Hssssssssssssss Ssssssssssssssss SSSGDDDDDDDD DDDDDDDDDDDD Yttttttttttttddd DDDDDDDocument8 pagesHssssssssssssss Ssssssssssssssss SSSGDDDDDDDD DDDDDDDDDDDD Yttttttttttttddd DDDDDDSean HuffNo ratings yet
- SDDDDDDDDDDD DDGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGGDocument2 pagesSDDDDDDDDDDD DDGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGGSean HuffNo ratings yet
- Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaagggg GGGGGGGGGGGGGDocument4 pagesAaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaagggg GGGGGGGGGGGGGSean HuffNo ratings yet
- Heat of CombustionDocument10 pagesHeat of CombustionSean HuffNo ratings yet
- XTXTTTTTTTTTTTTTTT TTTTTTTTTTTTTTTTTTT TXXXXXXXXXXXXXX XXXXXXXXXXXXXXX XXXXXXXXXXXXXXX XXXXXTTTTTTTTTTTTDocument3 pagesXTXTTTTTTTTTTTTTTT TTTTTTTTTTTTTTTTTTT TXXXXXXXXXXXXXX XXXXXXXXXXXXXXX XXXXXXXXXXXXXXX XXXXXTTTTTTTTTTTTSean HuffNo ratings yet
- Ssssssssssssssss Ssssssssssssssss SSSSGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GgggggggssssssDocument4 pagesSsssssssssssssss Ssssssssssssssss SSSSGGGGGGGGGG GGGGGGGGGGGGG GGGGGGGGGGGGG GgggggggssssssSean HuffNo ratings yet
- JCR Impact Factors List 2013Document281 pagesJCR Impact Factors List 2013vpisinarNo ratings yet
- Group Bsalvador Spain vs. Netherlands 3:00 Am MytDocument4 pagesGroup Bsalvador Spain vs. Netherlands 3:00 Am MytSean HuffNo ratings yet
- BBB BBBBBBDocument4 pagesBBB BBBBBBSean HuffNo ratings yet
- GGGGGGGGGGG GGGGGGGFCTDD DDDDDDDDDDD Dddjjjjjjjjjjjjjyy YyyyyyyyyyyyyDocument2 pagesGGGGGGGGGGG GGGGGGGFCTDD DDDDDDDDDDD Dddjjjjjjjjjjjjjyy YyyyyyyyyyyyySean HuffNo ratings yet
- CCCCCCCDDDDDD DDDDDDDDDDTTT TTTTTTTTTTTTTTTTTT TTTHHHHHHHHHH HHHHHHHHHHHH HHHHTTTTTTTTTTTTDocument2 pagesCCCCCCCDDDDDD DDDDDDDDDDTTT TTTTTTTTTTTTTTTTTT TTTHHHHHHHHHH HHHHHHHHHHHH HHHHTTTTTTTTTTTTSean HuffNo ratings yet
- Aaaaaaaaaaagg Ggggggggggaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaah HHHHHHHHHHHHDocument2 pagesAaaaaaaaaaagg Ggggggggggaaa Aaaaaaaaaaaaa Aaaaaaaaaaaaa Aaaaaaaaaaah HHHHHHHHHHHHSean HuffNo ratings yet
- Document summary yyyyyDocument2 pagesDocument summary yyyyySean HuffNo ratings yet
- PR 4Document3 pagesPR 4Sean HuffNo ratings yet
- Form 2 Chapter 8Document6 pagesForm 2 Chapter 8naza9775100% (4)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- BS 476-7-1997Document24 pagesBS 476-7-1997Ivan ChanNo ratings yet
- Red ProjectDocument30 pagesRed ProjectApoorva SrivastavaNo ratings yet
- Converting Units of Measure PDFDocument23 pagesConverting Units of Measure PDFM Faisal ChNo ratings yet
- Leibniz Integral Rule - WikipediaDocument70 pagesLeibniz Integral Rule - WikipediaMannu Bhattacharya100% (1)
- Daily Lesson Plan: Week DAY Date Class Time SubjectDocument3 pagesDaily Lesson Plan: Week DAY Date Class Time SubjectHasanah HassanNo ratings yet
- Multiple Choice Test - 66253Document2 pagesMultiple Choice Test - 66253mvjNo ratings yet
- Court Testimony-WpsDocument3 pagesCourt Testimony-WpsCrisanto HernandezNo ratings yet
- SQM-Crop Kit Pepper L-EnDocument96 pagesSQM-Crop Kit Pepper L-EnPavel Lilian100% (3)
- Senator Frank R Lautenberg 003Document356 pagesSenator Frank R Lautenberg 003Joey WilliamsNo ratings yet
- Econometrics IntroductionDocument41 pagesEconometrics IntroductionRay Vega LugoNo ratings yet
- Duah'sDocument3 pagesDuah'sZareefNo ratings yet
- 1000 Electronic Devices & Circuits MCQsDocument467 pages1000 Electronic Devices & Circuits MCQskibrom atsbha67% (3)
- Edwards 1999 Emotion DiscourseDocument22 pagesEdwards 1999 Emotion DiscourseRebeca CenaNo ratings yet
- Araminta Spook My Haunted House ExtractDocument14 pagesAraminta Spook My Haunted House Extractsenuthmi dihansaNo ratings yet
- 202002Document32 pages202002Shyam SundarNo ratings yet
- Assignment Chemical Bonding JH Sir-4163 PDFDocument70 pagesAssignment Chemical Bonding JH Sir-4163 PDFAkhilesh AgrawalNo ratings yet
- Modbus Quick StartDocument3 pagesModbus Quick StartNash JungNo ratings yet
- Listening LP1Document6 pagesListening LP1Zee KimNo ratings yet
- ATS - Contextual Theology SyllabusDocument4 pagesATS - Contextual Theology SyllabusAts ConnectNo ratings yet
- Jason A Brown: 1374 Cabin Creek Drive, Nicholson, GA 30565Document3 pagesJason A Brown: 1374 Cabin Creek Drive, Nicholson, GA 30565Jason BrownNo ratings yet
- Q-Win S Se QuickguideDocument22 pagesQ-Win S Se QuickguideAndres DennisNo ratings yet
- Needs and Language Goals of Students, Creating Learning Environments andDocument3 pagesNeeds and Language Goals of Students, Creating Learning Environments andapi-316528766No ratings yet
- North American Indians - A Very Short IntroductionDocument147 pagesNorth American Indians - A Very Short IntroductionsiesmannNo ratings yet
- Commonlit The Cask of AmontilladoDocument10 pagesCommonlit The Cask of Amontilladoapi-506044294No ratings yet
- Family Health Nursing Process Part 2Document23 pagesFamily Health Nursing Process Part 2Fatima Ysabelle Marie RuizNo ratings yet
- Module 3 in Oral Com 1Document20 pagesModule 3 in Oral Com 1Trisha DiohenNo ratings yet
- SMAW Product DevelopmentDocument9 pagesSMAW Product Developmenttibo bursioNo ratings yet
- Case DurexDocument3 pagesCase DurexGia ChuongNo ratings yet
- Business Policy FormulationDocument21 pagesBusiness Policy FormulationWachee Mbugua50% (2)
- Integrating GrammarDocument8 pagesIntegrating GrammarMaría Perez CastañoNo ratings yet