Professional Documents
Culture Documents
Cancer
Uploaded by
Richard S. RoxasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cancer
Uploaded by
Richard S. RoxasCopyright:
Available Formats
Int J Gynecol Obstet, 1993, 43: 305-312 International Federation of Gynecology
305 and Obstetrics
Synchronous dual primary ovarian and endometrial carcinomas*
M.L. Pearl** , C.M. Johnstona,
Departments Center. of Obstetrics Michigan and Gynecology (USA) und
T.S. Frankb
Pathology,
and J.A. Roberts
qf Gynecologic Oncology. University of Michigan Medical
Division
Ann Arbor,
(Received May 31st. 1993) (Revised and accepted July 29th.
1993)
Abstract
OBJECTIVES: The synchronous occurrence of carcinoma confined to the ovary and endometrium presents a diagnostic and therapeutic dilemma. These tumors have been variously staged as FIG0 Stage IIA ovarian carcinoma, Stage III endometrial carcinoma, or synchronous dual primary carcinomas. Accumulating evidence suggests such patients have a favorable outcome. This retrospective study was undertaken to review our experience with these fascinating tumors. METHODS: The clinical records and the pathologic jindings of 16 patients with synchronous dual primary ovarian and endometrial carcinomas were reviewed. RESULTS: The median age was 51 years. Abnormal uterine bleeding was the most common presenting symptom (70 %). All patients had Stage I ovarian and endometrial carcinomas. Fourteen patients (88%) had endometrioid carcinoma in both sites, while two patients (12%) had dissimilar histology. For 15 patients (94%), the grade of both tumors was identical. Only three (19 %) patients had myometrial invasion, with less than 50% involvement in each case. All patients underwent
Presented at the 4lst Annual Clinical Meeting of the American College of Obstetricians and Gynecologists. Washington, D.C. **Recipient of American Cancer Society Clinical Oncology Fellowship 92-101-l.
surgical staging, I1 (70%) of whom received adjuvant radiation or chemotherapy. The five patients treated with surgery alone had Grade I endometrioid tumors. The only relapse occurred in a patient with a clear cell component in both sites. No patient has died of disease. CONCLUSIONS: Patients with synchronous dual primary carcinomas appear to have a more favorable prognosis than that expected with Stage IIA ovarian or Stage III endometrial carcinoma (100% vs. 63 % or 42 % survival at 3 years, respectively). The excellent survival for patients with Grade 1 dual endometrioid tumors treated with surgery alone suggests that adjuvant therapy may not be necessary for this sub-group.
Keywords: Ovarian; ncy; Synchronous. Introduction
Endometrial;
Maligna-
The synchronous occurrence of carcinoma in the ovary and the endometrium is uncommon. The incidence of endometrial carcinoma in women with ovarian carcinoma ranges from 4.5 to 300/o,with the highest frequency occurring with endometrioid ovarian carcinoma [l-4]. Conversely, the incidence of ovarian carcinoma in women with endometrial carcinoma ranges from 2 to 8.5% [l-5].
Article
0020-7293/93/$06.00 0 1993 International Federation Printed and Published in Ireland
of Gynecology
and Obstetrics
306
Pearl et al,
The appropriate staging of these cases is controversial. Various authors have suggested staging such lesions as FIG0 Stage IIA ovarian carcinoma, Stage III endometrial carcinoma, or synchronous primary carcinomas [6-l I]. Classification of these tumors as dual primary carcinomas is relatively straightforward when the histology is dissimilar. However, such a designation is more difficult when the histology is similar or identical. Evidence is accumulating that suggests women with dual primary carcinomas have a favorable outcome. The survival for women with dual endometrioid carcinomas has been reported as 66-iOO % [6,8]. In contrast, the 5year survival is 45% for women with Stage II ovarian carcinoma [12], and 33-36% for women with Stage III endometrial carcinoma [13,14]. The therapeutic approach to these patients varies. Some authors suggest that surgical management alone may be adequate for selected patients [6,8,9], while others believe that adjuvant therapy is indicated [lo]. A wide variety of adjuvant therapies has been utilized, including radiation therapy, both external beam and brachytherapy, as well as a multitude of chemotherapy regimens. The purpose of this study was to review our experience with synchronous dual primary carcinomas of the ovary and endometrium.
Materials and methods
treatment and follow-up. The entation, pathologic findings were reviewed with regard to histology, depth of invasion, grade and stage. The patients were divided into three groups according to the histology of the ovarian and endometrial tumors [6]. Group A consisted of patients with similar endometrioid ovarian and endometrial histology (Fig. la and b). Group B consisted of patients with nonendometrioid but similar ovarian and endometrial histology. Group C consisted of patients with dissimilar ovarian and endometrial histology (Fig. 2a and b). All patients were re-staged according to surgical findings utilizing current FIG0 criteria [ 15,161. Staging was performed assuming the presence of synchronously occurring dual primary carcinomas. Statistical analysis was by chi-square test of homogeneity, with P < 0.05 considered significant.
Results
Twenty patients with the diagnosis of synchronous dual primary ovarian and endometrial carcinomas were treated by the Gynecologic Oncology Service at the University of Michigan Medical Center from 1980- 1991. Four patients were excluded from analysis on review: three had intra-abdominal spread of tumor precluding accurate classification as dual primary tumors, and one was lost to follow-up 1 month after initial surgery. The clinical records of the remaining 16 patients were abstracted to obtain information regarding their age, gravity, menopausal status, hormonal replacement therapy, presInt J Gynecol Obstet 43
The median age at presentation was 51 years (range: 39-65 yrs). Ten (63%) patients were post-menopausal, of which only two were on hormonal replacement therapy. There were 3 (19%) nulligravid women; the median gravity was 2.5 (range: O-6). The presenting complaint was abnormal bleeding in 10 (63%) patients, while 6 (37%) had a palpable adnexal mass. A total of 127 ovarian and 353 endometrial carcinomas were treated by our division during the study period. Synchronous occurrence of dual primary tumors occurred in 12.6% of the ovarian carcinoma patients and 4.5% of the endometrial carcinoma patients. All sixteen patients had Stage I ovarian carcinoma: eight (50%) were Stage IA, two were Stage IB (12%), and six were Stage IC (38%) (Table 1). Both patients with Stage IB tumors had identical histology in both ovaries. Five of the Stage IC patients had carcinoma arising in a focus of surface endometriosis, while two, including one with
Primarv
carcinomas
307
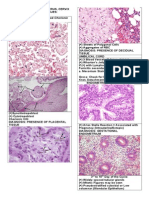
Fig. I.
Simultaneous
ovarian
and endometrial x 330).
carcinomas
of similiar histology.
Both the ovarian (a) and endometrial
neoplasms
(b) have an endometrioid
histology, characterized
by prominent
glands lined by columnar
cells with clear cytoplasm and stratifying,
round to oval nuclei (magnification
surface endometriosis, had intra-operative rupture of the ovarian cyst. The histology of the ovarian carcinoma was endometrioid in fifteen (94%) patients, two of which contained either a clear cell or adenosquamous component. The histology of the remaining carcinoma was transitional cell carcinoma. Twelve lesions were Grade 1 (75X), three were Grade 2 (19X), and one was Grade 3 (6%). All sixteen patients had Stage I endometrial carcinoma: 13 (81%) were Stage IA and 3 (19%) were Stage IB (Table 1). The histology
of the endometrial carcinoma was endometrioid in 15 (94%) patients, one of which contained a clear cell component. The histology of the remaining carcinoma was mucinous. Thirteen lesions were Grade 1 (8 1%) and three were Grade 2 (19%). In 15 patients (94X), the endometrial tumor was the same grade as the ovarian tumor. Each patient with myometrial involvement had less than 50% invasion into the myometrium. Twelve (75%) patients had endometrioid ovarian and endometrial carcinomas and were
Article
308
Pearl et al
Fig. 2.
Simultaneous ovarian and endometrial
carcinomas of divergent histology. The ovarian carcinoma (a) is of a transitional core without prominent rounded gland formation. In contrast, the endometrial x 330). nuclei similar to Fig. I (magnification
type car-
in which spindled cells surround a central fibrovascular
cinoma (b) is comprised of glands lined by cells with uniform,
classified as Group A. One (6%) patient had identical endometrioid ovarian and endometrial carcinomas containing a clear cell component and was classified as Group B. The remaining two (12%) patients had dissimilar ovarian and endometrial carcinomas: a transitional cell carcinoma and an endometrioid ovarian carcinoma with an adenosquamous component, both in conjunction with endometrioid endometrial carcinoma. These patients were classified as Group C. All patients underwent surgical staging,
Inr J Gynecol Obstet 43
consisting of cytologic washings, total abdominal hysterectomy, bilateral salpingooophorectomy, omentectomy and pelvic lymphadenectomy. Five (31%) patients were treated with surgery alone. One (6%) patient received a preoperative cesium implant. Three (19%) patients were treated with post-operative intra-peritoneal 32P. Four (25%) patients received post-operative external beam radiation therapy, either whole abdomen [3] or whole abdomen and whole pelvis [ 11. Five (31%) patients were treated with various post-
Primary
carcinomas
309
operative chemotherapy regimens: carboplatin; cisplatin/cyclophosphamide; melphalan/ medroxyprogesterone acetate; 5flurouracil/ vinblastine/medroxyprogesterone acetate. The only recurrence (6%) occurred in the woman whose endometrioid ovarian and endometrial carcinomas each contained a clear cell component. She received cisplatin/cyclophosphamide chemotherapy for pulmonary metastases 36 months after her initial surgery, followed by whole brain irradiation and leukovorin/FuDR/carboplatin chemotherapy for brain metastases 12 months later. She is currently alive with disease 62 months after presentation. With a median follow-up of 39 months, no patient has died of disease. Discussion The results of this study suggest that patients with synchronous dual primary ovarian and endometrial carcinomas have a favorable prognosis. The overall survival was loo%, with a median follow-up of 39 months. This is
markedly better than the extrapolated 63% or 42-65% 3-year survival for patients with Stage II ovarian [17] or Stage III endometrial carcinoma, respectively [ 13,141. The median age at presentation was 51 years, comparable to previous reports in which the median age ranged from 41 to 54 years [6,7,10,11]. Thus, it appears that patients with dual primary carcinoma tend to be lo-20 years younger than their counterparts with ovarian or endometrial carcinoma, in whom the median age at presentation is approximately 60 years [ 18,191. As expected given the early stage of the ovarian tumor, the majority of patients presented with abnormal bleeding from the endometrial tumor. Previous investigators have reported survival rates of 57-100% for Group A, with an average of 80% (Table 2) [6-lo]. The variation in survival was attributed to variation in the depth of myometrial invasion [6-IO]. Patients with deeply invasive (> 2/3) tumors appeared to have a significantly worse prognosis than did those whose tumors were less
Table 1. Clinical characteristics of patients with ovarian and endometrial carcinomas. Patient L.B. M.B. M.C. UC. J.D. J.G. G.H. L.K. J.K. J.E.K. E.K. S.L. M.E. I.M. J.M. J.Z. Ovary Stage IC IA IA IA IA IB IC IA IA IA IB IC IC IC IC IA corpus Stage IA IA IA IA IA IB IB IA IA IA IB IA IA IA IA IA Rx Grade I I I 1 2 I 1 1 I I 2 1 I 1 1 2 Hist. E E, CC E M E E E E E E E E E E E E Inv. ti 4 Q 6 6 < l/3 < l/2 4 6 4 < 113 + + o & f$ S, R S, R S S S, R3 S S, C S, C S S, C R*, S. C R4 S S, R3, C S. R4 S, R4 NED AWD NED NED NED NED NED NED NED NED NED NED NED NED NED NED 82 62 25 I9 3 102 I9 16 6 42 143 82 84 I44 80 42 Status Months
Grade 1 1 I I 2 1 I I I 3 2 I 1 1 I 2
Hist. E E, CC E E E E, AS E E E TC E E E E E E
E, endometrioid; CC, clear cell; M, mutinous; AS, adenosquamous; TC, transitional cell carcinoma; S, surgery; R , whole abdomen and whole pelvis irradiation; RZ, preoperative cesium irradiation; R3, whole abdomen irradiation; R4, intraperitoneal P3 ; C, chemotherapy; NED, no evidence of disease; AWD, alive with disease.
Article
310
Pearl et al.
Table 2. Reference
Survival. Group N A Group N B Group N C
Survival (X) 100
Survival ( %I) 45 25 100 100 50 100 53
Survival ( %I) 50 56 50 100 59
6 7 8 9 10 Pearl Total Group Group
16
I
I3 13 23 I2 78
100
77 100 57 100 83
II 4 2 2 IO I 30
2 9 4 2 I7
A: Endometrioid ovarian and endometrial histology. B: Non-endometrioid but similar ovarian and enhistology.
dometrial histology. Group C: Dissimilar ovarian and endometrial P < 0.05 vs. Groups B and C.
invasive (Table 3) [6-lo]. None of the patients in the present study had deeply invasive tumors, possibly contributing to the excellent survival rate. In contrast, survival rates of 25- 100% have been reported for patients in Group B, with an average of 52% (Table 2) [6-lo]. Similarly, the reported survival rates for patients in Group C are 50- lOO%, with an average of 53% (Table 2) [6-lo]. The worse prognosis in these patients, as compared with the patients in Group A, has been attributed to greater aggressiveness of the non-endometrioid histo-
logic types, which often present with deep myometrial or ovarian hilar invasion [6- lo]. Although the results for Groups B and C from the present study are comparable to previous studies, the numbers are too small to draw any meaningful conclusions. The etiology of these carcinomas is uncertain. Several investigators have proposed that the extended Mullerian system, comprising the ovarian epithelium, fallopian tube, uterine corpus and cervix may respond as a single morphologic unit to produce primary carcinomas in multiple sites [6,20]. Endometriosis has been considered a predisposing factor by some [11,21], but not all investigators [6,22]. Similarly, previous investigators have implicated estrogen exposure as a stimulus for malignant transformation [23], citing the welldocumented association between estrogen exposure and the development of endometrial carcinoma [24]. Determining whether synchronously occurring ovarian and endometrial carcinomas represent dual primary tumors or metastatic lesions from either site remains difficult and controversial. Several investigators have classified the lesions as dual primary tumors if the endometrial lesion was minimally invasive, well-differentiated, and less than 2 cm in diameter [2,25,26]. Some authors have sug-
Table 3. Reference
Depth of invasion Group < 213 N ( A,) Survival ( %I) 100 100 89 100 75 100 92 A
and survival. Group >2/3 N Survival ( %I) (I%>) I 4 3 7 I5 100 50 100 14 47 <2/3 N Survival (I%,) (I%,) 4 4 2 5 I I6 25 25 100 80 100 56 B > 213 N Survival ( %I) (1%)) 7 57 4 2 3 2 II 75 100 67 100 82 5 I 6 40 100 50 Group < 213 N Survival (1%)) ( %I) C > 213 N Survival (1%)) ( K)
6 7 8 9 IO Pearl Total
I5 I 9 IO I6 I3 64
5 I2 histology
20 42
Group A: Endometrioid ovarian and endometrial histology. Group B: Non-endometrioid but similar ovarian and endometrial Group C: Dissimilar ovarian and endometrial histology. P < 0.05 vs. Group A > 213. Inr J Gynecol Obstet 43
Primary carcinomas
3II
gested that the presence of dissimilar histologic subtypes indicates the lesions are primary, rather than metastatic, tumors [ 11,271. Still others have utilized the histologic grade, classifying Grade 1 and 2 lesions as dual primary, and Grade 3 lesions as metastatic, tumors [21]. Finally, the excellent survival for these patients has been used to support classifying these lesions as dual primary tumors [3,6,8,10]. All investigators agree that careful and extensive clinicopathologic evaluation is a prerequisite for accurate classificaton. The treatment of these tumors varies widely. In the present study, survival was excellent following surgical therapy with or without adjuvant therapy. There were no recurrences in the patients with Grade 1 endometrioid tumors treated with surgery alone, supporting previous reports that suggest adjuvant therapy may not be necessary for patients in this subgroup [6,7,9]. In contrast, patients with higher grade ovarian tumors are usually treated with adjuvant irradiation or chemotherapy [28], and this approach should be applied to such patients with ovarian and endometrial tumors. Finally, the nonendometrioid or dissimilar histologic types may be aggressive [6,8], and should be treated with adjuvant irradiation or chemotherapy. In conclusion, patients with synchronous dual primary ovarian and endometrial carcinomas appear to have a favorable prognosis. However, the currently available information is derived from small, retrospective studies. The definitive answer to the appropriate staging and treatment of these fascinating tumors requires a multicenter, randomized prospective trial. References Anneger JF,
Malkasian CID: Patterns of other neoplasm5 in patients with endometrial carcinoma. Cancer 48: 856, 1981. Scully RE: Tumours of ovary and maldeveloped gonads. Atlas of Tumour Pathology, Series II-Fast. 16. Armed Forces Institute of Pathology, Washington, 1980. Czernobilsky B, Silverman BD, Mikuta JJ: Endometrioid carcinoma of the ovary. Cancer 26: 1141, 1972.
5 6
Klemi KJ, Gronoos M: Endometrioid carcinoma of the ovary. A clinicopathologic, histochemical, and electronmicroscopic study. Obstet Gynecol 53: 572, 1979. Kottmeier HL: The diagnosis and treatment of ovarian malignancies. Arch Pathol 37: 51, 1965. Eifel P, Hendrickson M, Ross J, Ballon S, Martinez A, Kempson R: Simultaneous presentation of carcinoma involving the ovary and the uterine corpus. Cancer 50. 163, 1982. Piura B, Glezerman M: Synchronous carcinomas of endometrium and ovary. Gynecol Oncol 33: 261, 1989. Zaino RJ, Unger ER. Whitney C: Synchronous carcinomas of the uterine corpus and ovary. Gynecol Oncol
19: 329, 1984.
IO
II I2
I3
I4
I5 I6 I7 I8
19
20 21
22
Jambhekar NA, Sampat MB: Simultaneous endometrioid carcinoma of the uterine corpus and ovary: A clinicopathologic study of 15 cases. J Surg Oncol 37: 20, 1988. Montoya F, Martin M, Schneider J, Matia JC. Rodriguez-Escudero FJ: Simultaneous appearance of ovarian and endometrial carcinoma: a therapeutic challenge. Eur J Gynaecol Oncol 10: 135, 1989. Choo YC, Naylor B: Multiple primary neoplasms of the ovary and uterus. Int J Gynecol Obstet 20; 327, 1982. Richardson GS, Scully RE, Najamosama Nikrui, Nelson JH: Common epithelial cancer of the ovary. N Engl J Med 312: 415, 1985. Greven KM, Curran WJ, Whittington R, Fanning J. Randall ME, Wilder J, Peters AJ: Analysis of failure patterns in stage HI endometrial carcinoma and therapeutic implications. Int J Radiat Oncol Biol Phys 17: 35. 1989. Grigsby PW, Perez CA, Kuske RR, Kao MS, Galakatos AE: Results of therapy, analysis of failures, and prognostic factors for clinical and pathological stage III adenocarcinema of the endometrium. Gynecol Oncol27: 44, 1987. FIGO: Corpus cancer staging. Int J Gynecol Obstet 28: 190, 2989. Staging Announcement: FIG0 Cancer Committee: Gynecol Oncol 25: 383, 1986. Annual report on the results of treatment in gynecologic cancer. Volume 21. Int J Gynecol Obstet 38: 249, 1992. Gloeckler-Ries LA, Hankey BF, Edwards BK: Cancer Statistics Review 1973-1987. National Cancer Institute. Division of Cancer Prevention and Control, 1990. Yanick R, Ries LG. Yates JW: Ovarian cancer in the elderly: An analysis of Surveillence, Epidemiolgy and End Results Program data. Am J Obstet Gynecol 154: 639, 1986. Woodruff JD, Julian CG: Multiple malignancy in the upper genital tract. Am J Obstet Gynecol 103: 810, 1969. Cummings PA, Fox H, Langley FA: An electron microscopic study of the endometrioid adenocarcinoma of the ovary and a comparison of its tine structure with that of normal endometrium and adenocarcinoma of the endometrium. J Pathol 113: 165, 1979. Ulbright JM, Roth LM: Metastatic and independent cancers of the endometrium and ovary. A clinicopatholoigc study of 34 cases. Hum Pathol 16: 28, 1985.
Article
3 12 23
Pearl et al,
Silverman BB, O Neill RT, Mikuta JJ: Multiple malignant tumors associated with primary carcinoma of the ovary.
Surg Gynecol Obstet 134: 244, 1972.
Address for reprints:
M.L. Pearl
Department of Obstetrics and Gynecology Medical Professional Building Rm. D2202-0718 1500 E. Medical Center Drive University of Michigan Medical Center Ann Arbor, MI 48109-0718 USA
24 2s 26 27
28
Ziel HK: Estrogen s role in endometrial cancer. Obstet Gynecol 60: 509, 1982. Kurman RJ, Craig JM: Endometrioid and clear cell carcinoma of the ovary. Cancer 29: 1653, 1972. Dokerty MB: Primary and secondary ovarian adenoacanthoma: Surg Gynecol Obstet 99: 392, 1954: Matlock DL, Salem FA, Charles EH, Savage EW: Synchronous multiple neoplasms of the upper female genital tract. Gynecol Oncol 13: 271, 1982. Ozals RF, Rubin SC, Dembo AJ, Robboy SJ: Epithelial ovarian cancer. In: Principles and Practice of Gynecologic Oncology. (eds WJ Hoskins, CA Perez, RC Young), pp. 731-782. JB. Lippincott Co. Philadelphia, 1992.
Int J Gynecol Obstet 43
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- 14 1 1 Blood ConsumptionDocument3 pages14 1 1 Blood ConsumptionRichard S. RoxasNo ratings yet
- Evidence-Based Medicine: The Basics: Will Olmstadt, MS, MPHDocument14 pagesEvidence-Based Medicine: The Basics: Will Olmstadt, MS, MPHRichard S. RoxasNo ratings yet
- Things Needed2Document1 pageThings Needed2Richard S. RoxasNo ratings yet
- Critical+appraisal+RCT Su+May+LiewDocument34 pagesCritical+appraisal+RCT Su+May+LiewRichard S. RoxasNo ratings yet
- Villi: RD THDocument2 pagesVilli: RD THRichard S. RoxasNo ratings yet
- Management of Hyperglycemia in Type 2 Diabetes A Patient-Centered ApproachDocument16 pagesManagement of Hyperglycemia in Type 2 Diabetes A Patient-Centered ApproachThawatchai NakkaratniyomNo ratings yet
- DM Update 2012Document64 pagesDM Update 2012Richard S. RoxasNo ratings yet
- EDICDocument13 pagesEDICRichard S. RoxasNo ratings yet
- AccordDocument2 pagesAccordRichard S. RoxasNo ratings yet
- 35.5 Inches LengthDocument1 page35.5 Inches LengthRichard S. RoxasNo ratings yet
- AdvanceDocument5 pagesAdvanceRichard S. RoxasNo ratings yet
- UKPDSDocument6 pagesUKPDSRichard S. RoxasNo ratings yet
- Week 1 Diabetes Pathophysiology and Glycemic ControlDocument10 pagesWeek 1 Diabetes Pathophysiology and Glycemic ControlRichard S. RoxasNo ratings yet
- Week 1: Vaccines History of Vaccines: POLIO VACCINE: 1930'sDocument2 pagesWeek 1: Vaccines History of Vaccines: POLIO VACCINE: 1930'sRichard S. RoxasNo ratings yet
- NOVEMBER 16, 2013 12:00 PM-3:00 PM SVB Room 503: Week 1: Methods of ResearchDocument2 pagesNOVEMBER 16, 2013 12:00 PM-3:00 PM SVB Room 503: Week 1: Methods of ResearchRichard S. RoxasNo ratings yet
- Week 3: Vaccines RECOMBINANT DNA: Viral Subunit SummaryDocument3 pagesWeek 3: Vaccines RECOMBINANT DNA: Viral Subunit SummaryRichard S. RoxasNo ratings yet
- FertilizationDocument3 pagesFertilizationRichard S. RoxasNo ratings yet
- CGM Week 2: Interstitial Fluid & Insulin PumpsDocument3 pagesCGM Week 2: Interstitial Fluid & Insulin PumpsRichard S. RoxasNo ratings yet
- Medical-Surgical Nursing ReviewDocument90 pagesMedical-Surgical Nursing Reviewɹǝʍdןnos99% (312)
- Disaster Preparedness SyllabusDocument7 pagesDisaster Preparedness SyllabusRichard S. RoxasNo ratings yet
- Virology SyllabusDocument6 pagesVirology SyllabusRichard S. RoxasNo ratings yet
- Ob 1Document61 pagesOb 1Richard S. RoxasNo ratings yet
- Pharmacology Case StudyDocument4 pagesPharmacology Case StudyRichard S. RoxasNo ratings yet
- Excitation CouplingDocument3 pagesExcitation CouplingRichard S. RoxasNo ratings yet
- Ob Concept RMRDocument86 pagesOb Concept RMRRichard S. RoxasNo ratings yet
- Schedule of Activities 1st Sem '08-'09Document7 pagesSchedule of Activities 1st Sem '08-'09Richard S. RoxasNo ratings yet
- OB Sked Midterm 2008-09Document2 pagesOB Sked Midterm 2008-09Richard S. RoxasNo ratings yet
- Easy QuestionsDocument8 pagesEasy QuestionsRichard S. RoxasNo ratings yet
- The Nursing Process and Client TeachingDocument15 pagesThe Nursing Process and Client TeachingRichard S. RoxasNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Mola Hidatidosa JESDocument24 pagesMola Hidatidosa JESmember12dNo ratings yet
- Medicineand Medical Research. 1 (1) : 24-24.: Daftar PustakaDocument6 pagesMedicineand Medical Research. 1 (1) : 24-24.: Daftar Pustakaanon_31273229No ratings yet
- Template Evidence Tables: 1.1. Evidence Table of Systematic ReviewsDocument10 pagesTemplate Evidence Tables: 1.1. Evidence Table of Systematic ReviewsthelordhaniNo ratings yet
- Ovarian CancerDocument34 pagesOvarian CancerHealth Education Library for People100% (4)
- Renal Cell Carcinoma: DR Kalyan K Sarkar Ms FrcsedDocument38 pagesRenal Cell Carcinoma: DR Kalyan K Sarkar Ms FrcsedMichael ParkNo ratings yet
- Subungual Melanoma in 4-Year-Old BoyDocument50 pagesSubungual Melanoma in 4-Year-Old Boystefani83No ratings yet
- Ovary, Fallopian Tube, Primary Peritoneal Carcinoma TNM, 8th Ed - UpToDateDocument3 pagesOvary, Fallopian Tube, Primary Peritoneal Carcinoma TNM, 8th Ed - UpToDateSol anonimoNo ratings yet
- Advanced Imaging Modalities PDFDocument94 pagesAdvanced Imaging Modalities PDFJohnQ100% (1)
- Understanding Brain Tumors in 40 CharactersDocument46 pagesUnderstanding Brain Tumors in 40 CharactersJoyce Minerva Montero SamsonNo ratings yet
- Detection of Breast Cancer From Histopathology Image and Classifying Benign and Malignant State Using Machine LearningDocument16 pagesDetection of Breast Cancer From Histopathology Image and Classifying Benign and Malignant State Using Machine Learningiot forumNo ratings yet
- Mediastinal Tumors: Ana M. WrightDocument19 pagesMediastinal Tumors: Ana M. WrightSupriyati RahayuNo ratings yet
- Volume3 Issue4 Jul Aug No.329 665 667Document3 pagesVolume3 Issue4 Jul Aug No.329 665 667MadeKurniawanAsNo ratings yet
- Bone Tumor Seminar: Types & ManagementDocument80 pagesBone Tumor Seminar: Types & ManagementPATHMAPRIYA GANESANNo ratings yet
- Icam 1Document10 pagesIcam 1dusseldorf27No ratings yet
- Modern Biomarkers in Prostate Cancer Ppt-1Document25 pagesModern Biomarkers in Prostate Cancer Ppt-1FredNo ratings yet
- IMRT Part 2 BJRDocument6 pagesIMRT Part 2 BJRsusdoctorNo ratings yet
- Hyperthermia: Moderator: DR - Rashi Agrawal Presentor: DR - Shreebha HariDocument43 pagesHyperthermia: Moderator: DR - Rashi Agrawal Presentor: DR - Shreebha HariJeevan SunaNo ratings yet
- EAU Guidelines On: Penile CancerDocument38 pagesEAU Guidelines On: Penile Cancerhypebeast dopeNo ratings yet
- ORAL CANCER ADOD-2-110 Annals of Dentistry and Oral Disorders Ambarkova Oral CancerDocument2 pagesORAL CANCER ADOD-2-110 Annals of Dentistry and Oral Disorders Ambarkova Oral CancerVesna AmbarkovaNo ratings yet
- Nasopharyngeal Tumor Classification GuideDocument22 pagesNasopharyngeal Tumor Classification GuidegraceswanNo ratings yet
- Round Cell Tumors FinalDocument86 pagesRound Cell Tumors FinalKush Pathak100% (2)
- Invasive Ductal Carcinoma, Left Breast Status PostDocument37 pagesInvasive Ductal Carcinoma, Left Breast Status PostKayla SalvadorNo ratings yet
- Beta 2 MicroglobulinDocument6 pagesBeta 2 MicroglobulinMohamed FaizalNo ratings yet
- Molecular Epidemiologi CancerDocument10 pagesMolecular Epidemiologi CancerM NnNo ratings yet
- Pathology 5.05a CervixDocument6 pagesPathology 5.05a CervixDranreb Berylle MasangkayNo ratings yet
- Lesson PlanDocument21 pagesLesson PlanRoshni GorasiyaNo ratings yet
- Overview of The 8th Edition TNM Classification For Head and Neck Cancer - 1Document13 pagesOverview of The 8th Edition TNM Classification For Head and Neck Cancer - 1daniaNo ratings yet
- Malignant Small Round Cell TumorsDocument12 pagesMalignant Small Round Cell TumorsSaptarshi Ghosh100% (1)
- Lung Cancer 1Document44 pagesLung Cancer 1DR.OMAR ABDALLANo ratings yet
- Lung Cancer Translational and Emerging Therapies by Kishan J. PandyaDocument276 pagesLung Cancer Translational and Emerging Therapies by Kishan J. PandyaADEEL ARSALANNo ratings yet