Professional Documents
Culture Documents
E344 2013 Summer Solution Set 3: (B) What Role Does Each Component Play in The Forming and Firing Procedures?
Uploaded by
Firdhaus ZulkifleOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
E344 2013 Summer Solution Set 3: (B) What Role Does Each Component Play in The Forming and Firing Procedures?
Uploaded by
Firdhaus ZulkifleCopyright:
Available Formats
!"##$%&'"$()**+,$ (-.
)/0-1$(+/$"$
1. 13.21 (a) What are the three main components of a whiteware ceramic such as porcelain? (b) What role does each component play in the forming and firing procedures? Solution (a) The three components of a whiteware ceramic are clay, quartz, and a flux. (b) With regard to the role that each component plays: Quartz acts as a filler material. Clay facilitates the forming operation since, when mixed with water, the mass may be made to become either hydroplastic or form a slip. Also, since clays melt over a range of temperatures, the shape of the piece being fired will be maintained. The flux facilitates the formation of a glass having a relatively low melting temperature.
2. 13.23 Cite one reason why drying shrinkage is greater for slip cast or hydroplastic products that have smaller clay particles. Solution The reason that drying shrinkage is greater for products having smaller clay particles is because there is more particle surface area, and, consequently, more water will surround a given volume of particles. The drying shrinkage will thus be greater as this water is removed, and as the interparticle separation decreases.
3. 14.9 For a linear polymer molecule, the total chain length L depends on the bond length between chain atoms d, the total number of bonds in the molecule N, and the angle between adjacent backbone chain atoms !, as follows:
#" & L! " !Nd!#$%!% ( $ &' !!
Furthermore, the average end-to-end distance for a series of polymer molecules r in Figure 14.6 is equal to
(14.11)
r ! " !d !!
(14.12)
A linear polytetrafluoroethylene has a number-average molecular weight of 500,000 g/mol; compute average values of L and r for this material. Solution This problem first of all asks for us to calculate, using Equation 14.11, the average total chain length, L, for a linear polytetrafluoroethylene polymer having a number-average molecular weight of 500,000 g/mol. It is
necessary to calculate the degree of polymerization, DP, using Equation 14.6. For polytetrafluoroethylene, from Table 14.3, each repeat unit has two carbons and four flourines. Thus, m = 2(AC) + 4(AF)
= (2)(12.01 g/mol) + (4)(19.00 g/mol) = 100.02 g/mol and
M #$$%$$$!&'()* DP! " ! n ! " ! ! " !#$$$ m +$$,$-!&'()* !!
which is the number of repeat units along an average chain. Since there are two carbon atoms per repeat unit, there are two CC chain bonds per repeat unit, which means that the total number of chain bonds in the molecule, N, is
! bonds. Furthermore, assume that for single carbon-carbon bonds, d = 0.154 nm and ! = just (2)(5000) = 10,000
109 (Section 14.4); therefore, from Equation 14.11
#" & L! " !Nd!#$%! % ( $& ' !!
( " $%/ %+ " !#$%&%%%'#%($)*!+,'! *-.+!$ '- ! " !$0)*!+, ) # 0 &, !!
It is now possible to calculate the average chain end-to-end distance, r, using Equation 14.12 as
r ! " !d !!
N ! " !#$%&'(!)*+ &$,$$$! " !&'%(!)*
4. 14.11 Sketch portions of a linear polystyrene molecule that are (a) syndiotactic, (b) atactic, and (c) isotactic. Use two-dimensional schematics per footnote 8 of this chapter. Solution We are asked to sketch portions of a linear polystyrene molecule for different configurations (using twodimensional schematic sketches). (a) Syndiotactic polystyrene
(b) Atactic polystyrene
(c) Isotactic polystyrene
5. Draw a typical conformation of an average atactic polystyrene molecule for average molecule weights of: (a) 50,000 g/mole; and (b) 1500 g/mole. Note that different conformations can be formed because the main chain of polystyrene can rotate without breaking the C-C sigma bonds. Solution (a) (b)
6. 14.22 Explain briefly why the tendency of a polymer to crystallize decreases with increasing molecular weight. Solution The tendency of a polymer to crystallize decreases with increasing molecular weight because as the chains become longer it is more difficult for all regions along adjacent chains to align so as to produce the ordered atomic array.
7. 15.15 Briefly explain how each of the following influences the tensile or yield strength of a semicrystalline polymer and why: (a) Molecular weight (b) Degree of crystallinity (c) Deformation by drawing (d) Annealing of an undeformed material Solution (a) The tensile strength of a semicrystalline polymer increases with increasing molecular weight. This effect is explained by increased chain entanglements at higher molecular weights. (b) Increasing the degree of crystallinity of a semicrystalline polymer leads to an enhancement of the tensile strength. Again, this is due to enhanced interchain bonding and forces; in response to applied stresses, interchain motions are thus inhibited. (c) Deformation by drawing increases the tensile strength of a semicrystalline polymer. This effect is due to the highly oriented chain structure that is produced by drawing, which gives rise to higher interchain secondary bonding forces. Moreover, drawing orients the polymer moleculaes, so the loading is increasingly along the main chains rather than between the chains. Hence, the load is increwasingly carried by stronger intra-molecular primary bonds rather than weaker inter-molecular secondary bonds. (d) Annealing an undeformed semicrystalline polymer produces an increase in its tensile strength because there would be an increase in crystallinity.
8. 15.32 Of those polymers listed in Table 15.2, which polymer(s) would be best suited for use as ice cube trays? Why? Solution In order for a polymer to be suited for use as an ice cube tray it must have a glass-transition temperature below 0C. Of those polymers listed in Table 15.2 only low-density and high-density polyethylene, PTFE, and polypropylene satisfy this criterion.
9. 15.39 Cite the primary differences between addition and condensation polymerization techniques. Solution For addition polymerization, the reactant species have the same chemical composition as the monomer species in the molecular chain. This is not the case for condensation polymerization, wherein there is a chemical reaction between two or more monomer species, producing the repeating unit. There is often a low molecular weight by-product for condensation polymerization; such is not found for addition polymerization.
Solution
A. 7 Carbons, 2 Oxygens, 4 Hydrogens: 7 x 12 + 2 x 16 + 4 x 1 = 120 g/mole
B. nHBA = 280,00 g/mole/120 g/mole = 2333 nPE = 280,000 g/mole / 28 g/mole = 10,000 i.e. a molecule of PE would contain many more monomer units compared to a molecule of poly(HBA).
C. We can expect that the mechanical properties for these two specimens would be different, because the higher molecular weight sample would suffer many more entanglements than the lower molecular weight sample.
11. 6.4 A cylindrical specimen of a titanium alloy having an elastic modulus of 107 GPa (15.5 ! 106 psi) and an original diameter of 3.8 mm (0.15 in.) will experience only elastic deformation when a tensile load of 2000 N (450 lbf) is applied. Compute the maximum length of the specimen before deformation if the maximum allowable elongation is 0.42 mm (0.0165 in.).
Solution We are asked to compute the maximum length of a cylindrical titanium alloy specimen (before deformation) that is deformed elastically in tension. For a cylindrical specimen
# d0 & $ A" ! # !" % ( $2' !!
where d0 is the original diameter. Combining Equations 6.1, 6.2, and 6.5 and solving for l0 leads to
& d 0 )2 "l!E%!( + "l "l "l!E "l!E% d 02 '2* l" ! # ! ! = = !#! =! $ F # F 4F E A0 !!
(0.42 " 10 #3 m)(107 " 10 9 N / m 2 ) ( $)(3.8 " 10 #3 m) 2 "! (4)(2000 N ) !!
= 0.255 m = 255 mm (10.0 in.)
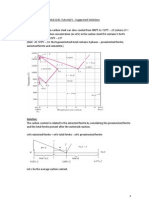
12. 6.25 Figure 6.21 shows the tensile engineering stressstrain behavior for a steel alloy. (a) What is the modulus of elasticity? (b) What is the proportional limit? (c) What is the yield strength at a strain offset of 0.002? (d) What is the tensile strength? Solution Using the stress-strain plot for a steel alloy (Figure 6.21), we are asked to determine several of its mechanical characteristics. (a) The elastic modulus is just the slope of the initial linear portion of the curve; or, from the inset and using Equation 6.10
" # "1 (200 # 0) MPa E! " 2 " " #$$! % !%$& !'()! " !#$$!*()! !+#9 ! % !%$, !-./0 $ # $ ( 0.0010 # 0 ) 2 1 !!
The value given in Table 6.1 is 207 GPa.
(b) The proportional limit is the stress level at which linearity of the stress-strain curve ends, which is
approximately 300 MPa (43,500 psi). (c) The 0.002 strain offset line intersects the stress-strain curve at approximately 400 MPa (58,000 psi). (d) The tensile strength (the maximum on the curve) is approximately 515 MPa (74,700 psi).
13. 7.1 To provide some perspective on the dimensions of atomic defects, consider a metal specimen that has a dislocation density of 104 mm-2. Suppose that all the dislocations in 1000 mm3 (1 cm3) were somehow removed and linked end to end. How far (in miles) would this chain extend? Now suppose that the density is increased to 1010 mm-2 by cold working. What would be the chain length of dislocations in 1000 mm3 of material? Solution The dislocation density is just the total dislocation length per unit volume of material (in this case per cubic millimeters). Thus, the total length in 1000 mm3 of material having a density of 104 mm-2 is just
("# $ %%&' )("### !%%( ) ) "#* %% ) "# $ !% ) +,' %!!
Similarly, for a dislocation density of 1010 mm-2, the total length is
("#"# $$ %& )("### !$$' ) ( "#"' $$ ( "#"# $ ( )*& ! " !"#) !$+ !!
14. 7.5 (a) Define a slip system. (b) Do all metals have the same slip system? Why or why not? Solution (a) A slip system is a crystallographic plane, and, within that plane, a direction along which dislocation motion (or slip) occurs. (b) All metals do not have the same slip system. The reason for this is that for most metals, the slip system will consist of the most densely packed crystallographic plane, and within that plane the most closely packed direction. This plane and direction will vary from crystal structure to crystal structure.
15. 7.23 (a) From the plot of yield strength versus (grain diameter)1/2 for a 70 Cu30 Zn cartridge brass, Figure 7.15, determine values for the constants "0 and ky in Equation 7.7. (b) Now predict the yield strength of this alloy when the average grain diameter is 1.0 ! 10-3 mm. Solution (a) Perhaps the easiest way to solve for "0 and ky in Equation 7.7 is to pick two values each of "y and d-1/2 from Figure 7.15, and then solve two simultaneous equations, which may be created. For example d-1/2 (mm) -1/2 4 12 "y (MPa) 75 175
The two equations are thus
"# $ "% & ' k y !! "#$ % "& ' "( k y !! !
Solution of these equations yield the values of
!
k y " #$%&!'()*++,#-$ !!
[#.#/ !012*++, ]
#-$
"0 = 25 MPa (3630 psi)
!
(b) When d = 1.0 # 10-3 mm, d-1/2 = 31.6 mm-1/2, and, using Equation 7.7,
" y " "# $ k y d %&'( !!
" #$% !&'() * +$,% !&'( (--) !! !
+.$
](/+,0 !--
1+.$
) " 2$3!&'(! !#0+4333 !567)
16. 7.32 Experimentally, it has been observed for single crystals of a number of metals that the critical resolved shear stress #crss is a function of the dislocation density $D as
" crss = " 0 + A # D
where #0 and A are constants. For copper, the critical resolved shear stress is 2.10 MPa (305 psi) at a dislocation
-3 density of 105 mm-2. If it is known that ! the value of A for copper is 6.35 ! 10 MPa-mm (0.92 psi-mm), compute the
"crss at a dislocation density of 107 mm-2.
Solution We are asked in this problem to compute the critical resolved shear stress at a dislocation density of 107 mm-2. It is first necessary to compute the value of the constant $0 (in the equation provided in the problem statement) from the one set of data as
" 0 = " crss # A $ D
= 2.10 !MPa " (6.35 # 10 "3 !"#$ % &&' () * !mm "2 = 0.092 !"#$ (13.3!psi) !! !
Now, the critical resolved shear stress may be determined at a dislocation density of 107 mm-2 as
""#$$ % " & ! ' ! A #D !!
" #$%$&'!()*+! , ! (-%./! " !0$ 1. !()* 1 22) 10 7 mm #2 " '$%' !()* !#'&'$!345+ !! ! !
Solution A. From the 0.2% offset method, "y = 160 MPa. "T = 185 MPa (the maximum stress on the stress-strain diagram) % Elongation to failure = 2.5% E = (160 0)/(0.01 0.002) = 20 GPa
(B) Indentation into cortical bone is less than that into trabecular bone, because according to Figure 5, trabecular bone has a much lower yield strength than cortical bone. (C)
L = 2 inch " (1 + (0.025 # 0.01)) = 2.03 inch , which corresponds to the total elongation minus the elastic
component.
18. B
19. B
20. B
21. B
22. C
23. E
24. C
25. A
26. D
27. E
You might also like
- Chronic Pain GuidelinesDocument56 pagesChronic Pain GuidelinesOporadhBiggan100% (1)
- Physical Security Audit Checklist PDFDocument3 pagesPhysical Security Audit Checklist PDFHendrawan StbNo ratings yet
- One Page AdventuresDocument24 pagesOne Page AdventuresPotato Knishes100% (1)
- M96SC05 Oleo StrutDocument6 pagesM96SC05 Oleo Strutchaumont12345No ratings yet
- Physical Therpay Protocols For Conditions of Neck RegionDocument74 pagesPhysical Therpay Protocols For Conditions of Neck Regionjrpsaavedra4599No ratings yet
- Chap-20 - Locomotion & MovementDocument52 pagesChap-20 - Locomotion & MovementMittal SavaniNo ratings yet
- Midterm Review SolutionsDocument16 pagesMidterm Review SolutionsKate SongNo ratings yet
- Practice Test AllDocument29 pagesPractice Test Alljraman2450% (2)
- Cyril Cromier, Frost & SullivanDocument24 pagesCyril Cromier, Frost & SullivanGaurav SahuNo ratings yet
- Interface / Interphase in Polymer NanocompositesFrom EverandInterface / Interphase in Polymer NanocompositesAnil N. NetravaliNo ratings yet
- Molecular Weight Calculations and Polymer PropertiesDocument19 pagesMolecular Weight Calculations and Polymer PropertiesPoovanaan Sathiya SeelanNo ratings yet
- MLE1101 - Tutorial 5 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 5 - Suggested SolutionsYin HauNo ratings yet
- Problems in Compos Mater Questions PG PDFDocument15 pagesProblems in Compos Mater Questions PG PDFJimmyFigueroaANo ratings yet
- AskelandPhuleNotes CH10PrintableDocument76 pagesAskelandPhuleNotes CH10PrintablejoshibecNo ratings yet
- 신소재과학 시험문제모음Document9 pages신소재과학 시험문제모음Hanjin SeoNo ratings yet
- Solutions to Selected Problems Chapter 7Document21 pagesSolutions to Selected Problems Chapter 7jacobtianNo ratings yet
- Assignment 2 SolDocument9 pagesAssignment 2 SolNickshan NahenthiramNo ratings yet
- Modeling Flow Through A Fixed Bed Packed ReactorDocument12 pagesModeling Flow Through A Fixed Bed Packed ReactorcharbelNo ratings yet
- Materials Science and Engineering Concept Check Part3 PDFDocument26 pagesMaterials Science and Engineering Concept Check Part3 PDF李宛芸No ratings yet
- HW2 Fall 2022Document3 pagesHW2 Fall 2022hamza balNo ratings yet
- CH 08Document8 pagesCH 08Ingi Abdel Aziz Srag0% (1)
- Chandamama, Mar 2011Document4 pagesChandamama, Mar 2011emediageNo ratings yet
- H.W 7Document9 pagesH.W 7Mirvat ShamseddineNo ratings yet
- Problem 15.1 A. To FindDocument8 pagesProblem 15.1 A. To FindRizwan KhanNo ratings yet
- EAT227 May 2019 ExamDocument5 pagesEAT227 May 2019 ExamΚωνσταντινος ΕυρουNo ratings yet
- Quiz 3 SolutionsDocument4 pagesQuiz 3 SolutionsM.USMAN BIN AHMED0% (1)
- Met 02023 Material Science IDocument13 pagesMet 02023 Material Science IlallyprabhNo ratings yet
- ch04 PDFDocument4 pagesch04 PDFAnderson Gomez CastroNo ratings yet
- 2017 AdvancesDocument8 pages2017 AdvancesAkshai Ashok KumarNo ratings yet
- Ceramics Engineering Exam Questions on Properties, Processing and ApplicationsDocument15 pagesCeramics Engineering Exam Questions on Properties, Processing and ApplicationsUtpal RoyNo ratings yet
- Closed-Book Practice-Ch 15 (2015!03!28)Document21 pagesClosed-Book Practice-Ch 15 (2015!03!28)JuanNo ratings yet
- Material Science and Engineering Chapter 4Document10 pagesMaterial Science and Engineering Chapter 4Jerc ZajNo ratings yet
- Fundamentals of Modern Manufacturing 6th Edition Groover Solutions ManualDocument5 pagesFundamentals of Modern Manufacturing 6th Edition Groover Solutions Manualduongnujl33q100% (29)
- Tute 9Document3 pagesTute 9vrm8000No ratings yet
- All Final ExamsDocument28 pagesAll Final Examsniikwabena36No ratings yet
- Problems Collection-2018Document19 pagesProblems Collection-2018calvinNo ratings yet
- TE 1997 and 2003 Course Oct 2009Document492 pagesTE 1997 and 2003 Course Oct 2009Ajay Solate0% (1)
- Question Paper of Summer Session 2022 23Document31 pagesQuestion Paper of Summer Session 2022 23moresachin7040No ratings yet
- Yes PleaseDocument14 pagesYes Pleasejaime1234No ratings yet
- MIT22 312F15 Final 2015Document4 pagesMIT22 312F15 Final 2015Angel de Jesus Lugo ZazuetaNo ratings yet
- Materials 100B Homework #3 SolutionsDocument5 pagesMaterials 100B Homework #3 SolutionsNiken Sylvia PuspitasariNo ratings yet
- 2Document2 pages2faizrummanNo ratings yet
- Of Ilietttbranes & Ion EschatqersDocument9 pagesOf Ilietttbranes & Ion Eschatqerskshitij shahNo ratings yet
- Tree ColumnDocument4 pagesTree ColumnDario P. YanezNo ratings yet
- 2022 - 350 Homework #1Document3 pages2022 - 350 Homework #1MeredithNo ratings yet
- Homework 3 With SolutionsDocument3 pagesHomework 3 With SolutionsDaniel RodriguezNo ratings yet
- EGR 2208 Assignment 3 - Composite Materials CalculationsDocument2 pagesEGR 2208 Assignment 3 - Composite Materials CalculationsIhsan Samoh เพี่อนดีดีNo ratings yet
- HW1 Fall 2022Document4 pagesHW1 Fall 2022hamza balNo ratings yet
- Materials selection & processing solutions for structural designDocument5 pagesMaterials selection & processing solutions for structural designputri nur shahidaNo ratings yet
- MT2soln PDFDocument5 pagesMT2soln PDFNickshan NahenthiramNo ratings yet
- Home Assignment Questions on Powder Metallurgy Properties & AnalysisDocument3 pagesHome Assignment Questions on Powder Metallurgy Properties & AnalysiszubbbuNo ratings yet
- Effect of SilaneDocument20 pagesEffect of SilaneVansala GanesanNo ratings yet
- Applied Chemistry ProblemsDocument24 pagesApplied Chemistry ProblemsKenneth C.LinojNo ratings yet
- Midterm Exam 1 - Summer 2016Document6 pagesMidterm Exam 1 - Summer 2016Tongtun Tuntun50% (2)
- MECH 321 Practice Problems Set #2Document4 pagesMECH 321 Practice Problems Set #2Sayyadh Rahamath BabaNo ratings yet
- Homework and Solutions - ch5 Ch6.IMSDocument18 pagesHomework and Solutions - ch5 Ch6.IMSHery RobiyantoroNo ratings yet
- Polymer Properties and Characterization"This title is concise (less than 40 characters) and SEO-optimized by including the main topic of the document ("Polymer Properties and CharacterizationDocument4 pagesPolymer Properties and Characterization"This title is concise (less than 40 characters) and SEO-optimized by including the main topic of the document ("Polymer Properties and CharacterizationOsama Aadil SaadiNo ratings yet
- Nwssu Final Exam 1Document1 pageNwssu Final Exam 1Axiel John Ray EscalaNo ratings yet
- Calculate Properties of Fiber-Reinforced CompositeDocument13 pagesCalculate Properties of Fiber-Reinforced CompositeNickshan NahenthiramNo ratings yet
- Exam 3 Material Science MATS 2001 UMN Fall 2012Document7 pagesExam 3 Material Science MATS 2001 UMN Fall 2012Zaki Smn100% (1)
- 2019 20-ExOrd19dic2019 V19dic SS Ing VfinalDocument8 pages2019 20-ExOrd19dic2019 V19dic SS Ing VfinalIñigoNo ratings yet
- Defect ProblemsDocument8 pagesDefect Problemsndreddy_pu100% (2)
- Carbon Nanomaterials for Advanced Energy Systems: Advances in Materials Synthesis and Device ApplicationsFrom EverandCarbon Nanomaterials for Advanced Energy Systems: Advances in Materials Synthesis and Device ApplicationsWen LuNo ratings yet
- Discrete-continuum Coupling Method to Simulate Highly Dynamic Multi-scale Problems: Simulation of Laser-induced Damage in Silica Glass, Volume 2From EverandDiscrete-continuum Coupling Method to Simulate Highly Dynamic Multi-scale Problems: Simulation of Laser-induced Damage in Silica Glass, Volume 2No ratings yet
- Nanostructured Polymer Membranes, Volume 1: Processing and CharacterizationFrom EverandNanostructured Polymer Membranes, Volume 1: Processing and CharacterizationNo ratings yet
- Tiziana BasementDocument1 pageTiziana BasementFirdhaus ZulkifleNo ratings yet
- Mass Mass (KG) R (M) MR Angle (Rad) Angle (Deg)Document5 pagesMass Mass (KG) R (M) MR Angle (Rad) Angle (Deg)Firdhaus ZulkifleNo ratings yet
- Tiziana Basement PDFDocument1 pageTiziana Basement PDFFirdhaus ZulkifleNo ratings yet
- Tiziana Basement PDFDocument1 pageTiziana Basement PDFFirdhaus ZulkifleNo ratings yet
- Li Course Audit FormDocument16 pagesLi Course Audit FormFirdhaus ZulkifleNo ratings yet
- ITunes Software LicenseDocument11 pagesITunes Software LicenseLilia CalugherNo ratings yet
- ReadmeDocument1 pageReadmeFirdhaus ZulkifleNo ratings yet
- E Last RomerDocument2 pagesE Last RomerFirdhaus ZulkifleNo ratings yet
- University of Selangor - Google MapsDocument1 pageUniversity of Selangor - Google MapsFirdhaus ZulkifleNo ratings yet
- Ci KFS3236 2012Document9 pagesCi KFS3236 2012Firdhaus ZulkifleNo ratings yet
- Unisel Industrial Training Report Duty FormDocument1 pageUnisel Industrial Training Report Duty FormFirdhaus ZulkifleNo ratings yet
- Thermodynamics 2 AssgDocument4 pagesThermodynamics 2 AssgFirdhaus ZulkifleNo ratings yet
- Application LetterDocument1 pageApplication LetterFirdhaus ZulkifleNo ratings yet
- PE1 q1 Mod6 ProperEtiquetteand-Safetyinthe-UseofFacilitiesEquip v1-ADMDocument12 pagesPE1 q1 Mod6 ProperEtiquetteand-Safetyinthe-UseofFacilitiesEquip v1-ADMelvira.raagas2No ratings yet
- Book 1Document100 pagesBook 1Devasyruc100% (1)
- Urinary System 1. List The Functions of The KidneysDocument6 pagesUrinary System 1. List The Functions of The KidneysheerNo ratings yet
- Common Safety Method GuidanceDocument66 pagesCommon Safety Method GuidanceDiego UngerNo ratings yet
- 1154ec108nanoelectronics PDFDocument3 pages1154ec108nanoelectronics PDFLordwin CecilNo ratings yet
- Two-day workshop budgetDocument2 pagesTwo-day workshop budgetVishwanath BaliNo ratings yet
- Moot CourtDocument7 pagesMoot CourtsushmaNo ratings yet
- SIDCSDocument8 pagesSIDCSsakshi suranaNo ratings yet
- PatternPro Variable Pitch GunDocument2 pagesPatternPro Variable Pitch GunVõ HòaNo ratings yet
- Greenhouse Effect: Greenhouse Gases and Their Impact On Global WarmingDocument9 pagesGreenhouse Effect: Greenhouse Gases and Their Impact On Global WarmingrabiulNo ratings yet
- Personal and Group Trainer Juan Carlos GonzalezDocument2 pagesPersonal and Group Trainer Juan Carlos GonzalezDidier G PeñuelaNo ratings yet
- Breading Guide To All FoodDocument1 pageBreading Guide To All FoodInno EspinaNo ratings yet
- Chemistry Code No. 1/2 Set: 3 Time Allowed: 3 Hours Maximum Marks: 100 General InstructionsDocument5 pagesChemistry Code No. 1/2 Set: 3 Time Allowed: 3 Hours Maximum Marks: 100 General InstructionsShalini KumariNo ratings yet
- Venkateshwara Institute of MedicalDocument10 pagesVenkateshwara Institute of Medicalbolhari070No ratings yet
- Ethics and Disasters: Patricia Reynolds Director, Bishopric Medical Library Sarasota Memorial Hospital Sarasota, FLDocument61 pagesEthics and Disasters: Patricia Reynolds Director, Bishopric Medical Library Sarasota Memorial Hospital Sarasota, FLChandra Prakash JainNo ratings yet
- Evaluation of Personal Dust Exposure of The Rice Mill Workers in AssamDocument10 pagesEvaluation of Personal Dust Exposure of The Rice Mill Workers in AssamIJAMTESNo ratings yet
- Community Medicine DissertationDocument7 pagesCommunity Medicine DissertationCollegePaperGhostWriterSterlingHeights100% (1)
- Rainwater Harvesting: Dr. Muhammad Anwar Baig Iese, Scee NUST H-12, IslamabadDocument30 pagesRainwater Harvesting: Dr. Muhammad Anwar Baig Iese, Scee NUST H-12, IslamabadTalha Bin UmeedNo ratings yet
- Escala de Violencia e Índice de SeveridadDocument11 pagesEscala de Violencia e Índice de SeveridadpsiserviciosprofesioNo ratings yet
- Weekly Home Learning Plan: Grade 8 - Quarter 2. Week 7Document3 pagesWeekly Home Learning Plan: Grade 8 - Quarter 2. Week 7Danmer Jude TorresNo ratings yet
- Home & Garden 2015Document32 pagesHome & Garden 2015The Standard NewspaperNo ratings yet
- SinogramDocument2 pagesSinogramNguyễn Thành CôngNo ratings yet
- Ra 9520 Chapter VDocument8 pagesRa 9520 Chapter VLorribelle OcenarNo ratings yet