Professional Documents
Culture Documents
Etiology and Pathogenesis Four
Uploaded by
egimaruCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Etiology and Pathogenesis Four
Uploaded by
egimaruCopyright:
Available Formats
ETIOLOGY AND PATHOGENESIS Four species of the genus Plasmodium cause nearly all malarial nfections in humans (although
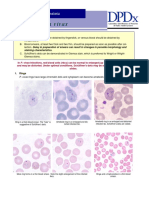
rare infections involve species normally affecting other primates). These are P. falciparum, P. vivax, P. ovale, and P. malariae (Table 195-1). Almost all deaths are caused by falciparum malaria. Human infection begins when a female anopheline mosquito inoculates plasmodial sporozoites from its salivary gland during a blood meal (Fig. 195-1). These microscopic motile forms of the malarial parasite are carried rapidly via the bloodstream to the liver, where they invade hepatic parenchymal cells and begin a period of asexual reproduction. By this amplification process (known as intrahepatic or preerythrocytic schizogony or merogony), a single sporozoite eventually may produce 10,000 to _30,000 daughter merozoites. The swollen liver cell eventually bursts, discharging motile merozoites into the bloodstream. These then invade the red blood cells (RBCs) and multiply 6- to 20-fold every 48 to 72 h. When the parasites reach densities of _50/_L of blood, the symptomatic stage of the infection begins. In P. vivax and P. ovale infections, a proportion of the intrahepatic forms do not divide immediately but remain dormant for a period ranging from 3 weeks to a year or longer before reproduction begins. These dormant forms, or hypnozoites, are the cause of the relapses that characterize infection with these two species. After entry into the bloodstream, merozoites rapidly invade erythrocytes and become trophozoites. Attachment is mediated via a specific erythrocyte surface receptor. In the case of P. vivax, this receptor is related to the Duffy blood-group antigen Fya or Fyb. Most West Africans and people with origins in that region carry the Duffy-negative FyFy phenotype and are therefore resistant to P. vivax malaria. During the early stage of intraerythrocytic development, the small ring forms of the four parasitic species appear similar under light microscopy. As the trophozoites enlarge, species-specific characteristics become evident, pigment becomes visible, and the parasite assumes an irregular or ameboid shape. By the end of the 48-h intraerythrocytic life cycle (72 h for P. malariae), the parasite has consumed nearly all the hemoglobin and grown to occupy most of the RBC. It is now called a schizont. Multiple nuclear divisions have taken place (shizogony or merogony), and the RBC ruptures to release 6 to 30 daughter merozoites, each potentially capable of invading a new RBC and repeating the cycle. The disease in human beings is caused by the direct effects of RBC invasion and destruction by the asexual parasite and the hosts reaction. After a series of asexual cycles ( P. falciparum) or immediately after release from the liver ( P.vivax, P. ovale, P. malariae), some of the parasites develop into morphologically distinct, long-lived sexual forms (gametocytes) that can transmit malaria. After being ingested in the blood meal of a biting female anopheline mosquito, the male and female gametocytes form a zygote in the insect s midgut. This zygote matures into an ookinete, which penetrates and encysts in the mosquitos gut wall. The resulting oocyst expands by asexual division until it bursts to liberate myriad motile sporozoites, which then migrate in the hemolymph to the salivary gland of the mosquito to await inoculation into another human at the next feeding. ERYTHROCYTE CHANGES IN MALARIA After invading an erythrocyte, the growing malarial parasite progressively consumes and degrades intracellular proteins, principally hemoglobin. The potentially toxic heme is polymerized to biologically inert hemozoin, or malaria pigment. The parasite also alters the RBC membrane by changing its transport properties, exposing cryptic surface antigens, and inserting new parasitederived proteins. The RBC becomes more irregular in shape, more antigenic, and less deformable. In P. falciparum infections, membrane protuberances appear on the erythrocytes surface toward the end of the first 24 h of the asexual cycle. These knobs extrude a high-molecularweight, antigenically variant, strain-specific, adhesive protein (PfEMP1) that mediates attachment to receptors on venular and capillary endothelium an event termed cytoadherence. Several vascular receptors have been identified, of which intercellular adhesion molecule 1 is probably the most important in the brain, chondroitin sulfate B in the placenta, and CD36 in most other organs. Thus the infected erythrocytes stick inside
the small blood vessels. At the same stage, these P. falciparuminfected RBCs may also adhere to uninfected RBCs to form rosettes and to other parasitized erythrocytes (agglutination). The processes of cytoadherence, rosetting, and agglutination are central to the pathogenesis of falciparum malaria. They result in the sequestration of RBCs containing mature forms of the parasite in vital organs (particularly the brain), where they interfere with microcirculatory flow and metabolism. Sequestered parasites continue to develop out of reach of the principal host defense mechanism: splenic processing and filtration. As a consequence, only the younger ring forms of the asexual parasites are seen circulating in the peripheral blood in falciparum malaria, and the level of peripheral parasitemia underestimates the true number of parasites within the body. Severe malaria is also associated with reduced deformability of the uninfected erythrocytes, which compromises their passage through the partially obstructed capillaries and venules and shortens RBC survival. In the other three (benign) malarias, sequestration does not occur, and all stages of the parasites development are evident on peripheralblood smears. Whereas P. vivax, P. ovale, and P. malariae show a marked predilection for either young RBCs (P. vivax, P. ovale) or old cells (P. malariae) and produce a level of parasitemia seldom _2%, P. falciparum can invade erythrocytes of all ages and may be associated with very high levels of parasitemia. HOST RESPONSE Initially, the host responds to plasmodial infection by activating nonspecific defense mechanisms. Splenic immunologic and filtrative clearance functions are augmented in malaria, and the removal of both parasitized and uninfected erythrocytes is accelerated. The parasitized cells escaping splenic removal are destroyed when the schizont ruptures. The material released induces the activation of macrophages and the release of proinflammatory mononuclear cellderived cytokines, which cause fever and exert other pathologic effects. Temperatures of _40_C damage mature parasites; in untreated infections, the effect of such temperatures is to further synchronize the parasitic cycle, with eventual production of the regular fever spikes and rigors that originally served to characterize the different malarias. These regular fever patterns (tertian, every 2 days; quartan, every 3 days) are seldom seen today in patients who receive prompt and effective antimalarial treatment. The geographic distributions of sickle cell disease, thalassemia, and glucose-6-phosphate dehydrogenase (G6PD) deficiency closely resemble that of malaria before the introduction of control measures. This observation suggests that these genetic disorders confer protection against death from falciparum malaria. For example, HbA/S heterozygotes (sickle cell trait) have a sixfold reduction in the risk of dying from severe falciparum malaria. This decrease in risk appears to be related to impaired parasite growth at low oxygen tensions. Parasite multiplication in HbA/E heterozygotes is reduced at high parasite densities. In Melanesia, children with _-thalassemia appear to have more frequent malaria (both vivax and falciparum) in the early years of life, and this pattern of infection appears to protect against severe disease. In Melanesian ovalocytosis, rigid erythrocytes resist merozoite invasion, and the intraerythrocytic milieu is hostile. The specific immune response to malaria eventually controls the infection and, with exposure to sufficient strains, confers protection from high-level parasitemia and disease but not from infection. As a result of this state of infection without illness (premunition), asymptomatic parasitemia is common among adults and older children living in regions with stable and intense transmission (i.e., holo- or hyperendemic areas). Immunity is mainly specific for both the species and the strain of infecting malarial parasite. Both humoral immunity and cellular immunity are necessary for protection, but the mechanisms of each are incompletely understood (Fig. 195-1). Immune individuals have a polyclonal increase in serum levels of IgM, IgG, and IgA, although much of this antibody is unrelated to protection. Antibodies to a variety of parasitic antigens presumably act in concert to limit in vivo replication of the parasite. In the case of falciparum malaria, the most important of these antigens is the variant protein PfEMP1 mentioned above. Passively transferred IgG from immune adults has been shown to reduce
levels of parasitemia in children, and passive transfer of maternal antibody contributes to the relative protection of infants from severe malaria in the first months of life. This complex immunity to disease declines when a person lives outside an endemic area for several months or longer. Several factors retard the development of cellular immunity to malaria. These factors include the absence of major histocompatibility antigens on the surface of infected RBCs, which precludes direct T cell recognition; malaria antigen specific immune unresponsiveness; and the enormous strain diversity of malarial parasites along with the ability of the parasites to express variant immunodominant antigens on the erythrocyte surface that change during the period of infection. Strain diversity also has an impact on the heterogeneity of the humoral antibody response. Immunity to all strains is never achieved. Parasites may persist in the blood for months (or, in the case of P. malariae, for many years) if treatment is not given. The complexity of the immune response in malaria, the sophistication of the parasites evasion mechanisms, and the lack of a good in vitro correlate with clinical immunity have all slowed progress toward an effective vaccine. COMPLICATIONS. Primary infections with dengue fever and dengue-like diseases are usually self-limited and benign. Fluid and electrolyte losses, hyperpyrexia, and febrile convulsions are the most frequent complications in infants and young children. Epistaxis, petechiae, and purpuric lesions are uncommon but may occur at any stage. Swallowed blood from epistaxis, vomited or passed by rectum, may be erroneously interpreted as gastrointestinal bleeding. In adults and possibly in children, underlying conditions may lead to clinically significant bleeding. Convulsions may occur during high temperature, especially with chikungunya fever. Infrequently, after the febrile stage, prolonged asthenia, mental depression, bradycardia, and ventricular extrasystoles may occur in children. In endemic areas, dengue hemorrhagic fever should be suspected in children with a febrile illness suggestive of dengue fever who experience hemoconcentration and thrombocytopenia. PROGNOSIS. The prognosis of dengue fever may be adversely affected by passively acquired antibody or by prior infection with a closely related virus that predisposes to development of dengue hemorrhagic fever. Dengue Hemorrhagic Fever. Death has occurred in 4050% of patients with shock, but with adequate intensive care deaths should occur in <1% of cases. Survival is directly related to early and intense supportive treatment. Infrequently, there is residual brain damage caused by prolonged shock or occasionally by intracranial hemorrhage. Dengue Hemorrhagic Fever. Differentiation between dengue fever and dengue hemorrhagic fever is difficult early in the course of illness. A relatively mild 1st phase with abrupt onset of fever, malaise, vomiting, headache, anorexia, and cough is followed after 25 days by rapid clinical deterioration and collapse. In this 2nd phase, the patient usually has cold, clammy extremities, a warm trunk, flushed face, diaphoresis, restlessness, irritability, and mid-epigastric pain. Frequently, there are scattered petechiae on the forehead and extremities; spontaneous ecchymoses may appear, and easy bruising and bleeding at sites of venipuncture are common. A macular or maculopapular rash may appear, and there may be circumoral and peripheral cyanosis. Respirations are rapid and often labored. The pulse is weak, rapid, and thready and the heart sounds faint. The liver may enlarge to 46 cm below the costal margin and is usually firm and somewhat tender. Approximately 20 30% of cases of dengue hemorrhagic fever are complicated by shock (dengue shock syndrome). Fewer than 10% of patients have gross ecchymosis or gastrointestinal bleeding, usually after a period of uncorrected shock. After a 24 36 hr period of crisis, convalescence is fairly rapid in the children who recover. The temperature may return to normal before or during the stage of shock. Bradycardia and ventricular extrasystoles are common during convalescence. The World Health Organization criteria for dengue hemorrhagic fever are fever, minor or major hemorrhagic manifestations, thrombocytopenia (100,000/mm3), and objective evidence of increased capillary permeability (hematocrit increased by 20%), serosal effusion (by chest radiography or ultrasonography), or hypoalbuminemia. Dengue shock syndrome criteria include those for dengue hemorrhagic fever as well as hypotension or narrow pulse pressure (20 mm Hg).
COMPLICATIONS Malaria Cerebral malaria is a serious complication of P. falciparum infection that is most common among children and nonimmune adults. It usually develops after the patient has been ill for several days and may develop
precipitously. Cerebral malaria is associated with a fatality rate of 20 40% but rarely causes long-term sequelae if treated appropriately. As with other complications, cerebral malaria is more likely among patients with parasitemia of >5%. The symptoms always include decreased level of consciousness and range in severity from drowsiness and severe headache to confusion, delirium, hallucinations, or deep coma. Physical findings may be normal or may include fever to 106108F, seizures, muscular twitching, rhythmic movement of the head or extremities, contracted or unequal pupils, retinal hemorrhages, hemiplegia, absent or exaggerated deep tendon reflexes, and a positive Babinski sign. Lumbar puncture reveals increased pressure and cerebrospinal fluid protein with minimal or no pleocytosis and a normal glucose concentration. There are no specific electroencephalographic findings with cerebral malaria. Renal failure is a common complication of severe P. falciparum malaria and results from deposition of hemoglobin in renal tubules, decreased renal blood flow, and acute tubular necrosis. Blackwater fever is a clinical syndrome that consists of severe hemolysis, hemoglobinuria, and renal failure. It is a rare complication that occurs when the combination of antibody directed against parasite-laden erythrocytes and complement result in severe hemolytic anemia, hemoglobinuria, oliguria, and jaundice. Renal failure usually requires peritoneal dialysis or hemodialysis. Pulmonary edema may occur several days after therapy has begun and is commonly associated with excessive intravenous therapy. It can develop rapidly and may be fatal. Thus, great care should be taken not to overhydrate patients with P. falciparum malaria. Hypoglycemia is a complication of malaria that is more common in children, pregnant women, and patients receiving quinine therapy. Patients may have a decreased level of consciousness that can be confused with cerebral malaria. Hypoglycemia is associated with increased mortality and neurologic sequelae. Thrombocytopenia is a common complication of P. falciparum and P. vivax malaria. Although significant bleeding is uncommon without DIC, platelet counts can decrease to 10,000 20,000/mm3. Splenic rupture is a rare complication that may occur with acute infection owing to any malaria species. Splenic rupture can occur spontaneously but is usually the result of trauma that includes overly vigorous palpation on physical examination. It causes severe internal hemorrhage and may result in death if removal of the spleen and blood transfusion are not performed in a timely manner. Algid malaria is a rare form of P. falciparum malaria that occurs with overwhelming infection, hypotension, hypothermia, rapid weak pulse, shallow breathing, pallor, and vascular collapse. Death may occur within a few hours. Among children living in endemic areas, malarial attacks adversely affect the educational attainment of the school-child. Prevention of attacks significantly improves educational attainment. Perubahan patofisiologi pada malaria terutama berhubungan dengan gangguan aliran darah setempat sebagai akibat melekatnya eritrosit yang mengandung parasit pada endotelium kapiler. Perubahan ini cepat reversibel pada mereka yang dapat tetap hidup (survive). Peran beberapa mediator humoral masih belum pasti, tetapi mungkin terlibat dalam patogenesis terjadinya demam dan peradangan. Skizogoni eksoeritrositik mungkin dapat menyebabkan reaski leukosit dan fagosit, sedangkan sporozoit dan gametosit tidak menimbulkan perubahan patofisiologik Patofisiologi malaria adalah multifaktorial dan mungkin berhubungan dengan hal-hal sebagai berikut : a. Penghancuran eritrosit. Penghancuran eritrosit ini tidak saja dengan pecahnya eritrosit yang mengandung parasit, tetapi juga oleh fagositosis eritrosit yang mengandung parasit dan yang tidak mengandung parasit, sehingga menyebabkan anemia dan anoksia jaringan. Dengan hemolisis intra vaskular yang berat, dapat terjadi hemoglobinuria (blackwater fever) dan dapat mengakibatkan gagal ginjal. b. Mediator endotoksin-makrofag. Pada saat skizogoni, eirtosit yang mengandung parasit memicu makrofag yang sensitif endotoksin untuk melepaskan berbagai mediator yang berperan dalam perubahan patofisiologi malaria. Endotoksin tidak terdapat pada parasit malaria, mungkin berasal dari rongga saluran cerna. Parasit malaria itu sendiri dapat melepaskan faktor neksoris tumor (TNF). TNF adalah suatu monokin , ditemukan dalam darah hewan dan manusia yang terjangkit parasit malaria. TNF dan sitokin lain yang berhubungan, menimbulkan demam, hipoglimeia dan sindrom penyakit pernafasan pada orang dewasa (ARDS = adult respiratory distress syndrome) dengan sekuestrasi sel neutrofil dalam pembuluh darah paru. TNF dapat juga menghancurkan plasmodium falciparum in vitro dan dapat meningkatkan perlekatan eritrosit yang dihinggapi parasit pada endotelium kapiler. Konsentrasi TNF dalam serum pada anak dengan malaria falciparum akut berhubungan langsung dengan mortalitas, hipoglikemia, hiperparasitemia dan beratnya penyakit. c. Sekuestrasi eritrosit yang terinfeksi. Eritrosit yang terinfeksi plasmodium falciparum stadium lanjut dapat membentuk tonjolan-tonjolan (knobs) pada permukaannya. Tonjolan tersebut mengandung antigen malaria dan bereaksi dengan antibodi malaria dan berhubungan dengan afinitas eritrosit yang mengandung plasmodium falciparum terhadap endotelium kapiler darah dalam alat dalam, sehingga skizogoni berlangsung di sirkulasi alat
dalam, bukan di sirkulasi perifer. Eritrosit yang terinfeksi, menempel pada endotelium kapiler darah dan membentuk gumpalan (sludge) yang membendung kapiler dalam alam-alat dalam. Protein dan cairan merembes melalui membran kapiler yang bocor (menjadi permeabel) dan menimbulkan anoksia dan edema jaringan. Anoksia jaringan yang cukup meluas dapat menyebabkan kematian. Protein kaya histidin P. falciparum ditemukan pada tonjolan-tonjolan tersebut, sekurang-kurangnya ada empat macam protein untuk sitoaherens eritrosit yang terinfeksi plasmodium P. falciparum. 5 Patogenesis Malaria Patogenesis malaria akibat dari interaksi kompleks antara parasit, inang dan lingkungan. Patogenesis lebih ditekankan pada terjadinya peningkatan permeabilitas pembuluh darah daripada koagulasi intravaskuler. Oeleh karena skizogoni menyebabkan kerusakan eritrosit maka akan terjadi anemia. Beratnya anemi tidak sebanding dengan parasitemia menunjukkan adanya kelainan eritrosit selain yang mengandung parasit. Hal ini diduga akibat adanya toksin malaria yang menyebabkan gangguan fungsi eritrosit dan sebagian eritrosit pecah melalui limpa sehingga parasit keluar. Faktor lain yang menyebabkan terjadinya anemia mungkin karena (6) terbentuknya antibodi terhadap eritrosit. Limpa mengalami pembesaran dan pembendungan serta pigmentasi sehingga mudah pecah. Dalam limpa dijumpai banyak parasit dalam makrofag dan sering terjadi fagositosis dari eritrosit yang terinfeksi maupun (6) yang tidak terinfeksi. Pada malaria kronis terjadi hyperplasia dari retikulosit diserta peningkatan makrofag. Pada malaria beratm mekanisme patogenesisnya berkaitan dengan invasi merozoit ke dalam eritrosit sehingga menyebabkan eritrosit yang mengandung parasit mengalami perubahan struktur danmbiomolekular sel untuk mempertahankan kehidupan parasit. Perubahan tersebut meliputi mekanisme, diantaranya (8) transport membran sel, sitoadherensi, sekuestrasi dan resetting . Sitoadherensi merupakan peristiwa perlekatan eritrosit yang telah terinfeksi P. falciparum pada reseptor di bagian endotelium venule dan kapiler. Selain itu eritrosit juga dapat melekat pada eritrosit yang tidak (4) terinfeksi sehingga terbentuk roset. . Resetting adalah suatu fenomena perlekatan antara sebuah eritrosit yang mengandung merozoit matang yang diselubungi oleh sekitar 10 atau lebih eritrosit non parasit, sehingga berbentu seperti bunga. Salah satu faktor yang mempengaruhi terjadinya resetting adalah golongan darah dimana terdapatnya antigen golongan (4,8) darah A dan B yang bertindak sebagai reseptor pada permukaan eritrosit yang tidak terinfeksi. Menurut pendapat ahli lain, patogenesis malaria adalah multifaktorial dan berhubungan dengan hal-hal sebagai berikut: 1. Penghancuran eritrosit Fagositosis tidak hanya pada eritrosit yang mengandung parasit tetapi juga terhadap eritrosit yang tidak mengandung parasit sehingga menimbulkan anemia dan hipoksemia jaringan. Pada hemolisis intravascular (9) yang berat dapat terjadi hemoglobinuria (black white fever) dan dapat menyebabkan gagal ginjal . 2. Mediator endotoksin-makrofag Pada saat skizogoni, eritrosit yang mengandung parasit memicu makrofag yang sensitive endotoksin untuk melepaskan berbagai mediator. Endotoksin mungkin berasal dari saluran cerna dan parasit malaria sendiri dapat melepaskan faktor nekrosis tumor (TNF) yang merupakan suatu monokin, ditemukan dalam peredaran darah manusia dan hewan yang terinfeksi parasit malaria. TNF dan sitokin dapat menimbulkan demam, (9) hipoglikemia, dan sndrom penyakit pernapasan pada orang dewasa . 3. Sekuestrasi eritrosit yang terluka Eritrosit yang terinfeksi oleh Plasmodium dapat membentuk tonjolan-tonjolan (knobs) pada permukaannya. Tonjolan tersebut mengandung antigen dan bereaksi dengan antibodi malaria dan berhubungan dengan afinitas eritrosit yang mengandung parasit terhadap endothelium kapiler alat dalam, sehingga skizogoni berlangsung di sirkulasi alat dalam. Eritrosit yang terinfeksi menempel pada endothelium dan membentuk gumpalan yang mengandung kapiler yang bocor dan menimbulkan anoksia dan edema (9) jaringan .
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Fluids and ElectrolytesDocument2 pagesFluids and ElectrolytesegimaruNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Asa Physical Status Classification SystemDocument1 pageAsa Physical Status Classification SystemegimaruNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Fluids and Electrolytes: Intensive Care Nursery House Staff ManualDocument6 pagesFluids and Electrolytes: Intensive Care Nursery House Staff ManualzviviNo ratings yet
- Inti Patologis Pada Gangguan Kepribadian Menghindar Atau Avoidant Personality DisorderDocument1 pageInti Patologis Pada Gangguan Kepribadian Menghindar Atau Avoidant Personality DisorderegimaruNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Everything You Need To Know About MolluscumDocument1 pageEverything You Need To Know About MolluscumegimaruNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Laboratory Guidance and Diagnostic TestingDocument4 pagesLaboratory Guidance and Diagnostic TestingegimaruNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Gambaran Klinis Demam Tifoid Bervariasi Dari GejalaDocument3 pagesGambaran Klinis Demam Tifoid Bervariasi Dari GejalaegimaruNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Career DevelopmentDocument3 pagesCareer DevelopmentegimaruNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Child's blurred vision disrupts brain developmentDocument2 pagesChild's blurred vision disrupts brain developmentGita ChrisantyNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Sedentary Life Style Fisiologi Olahraga IIDocument32 pagesSedentary Life Style Fisiologi Olahraga IIegimaruNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Cerebral Palsy Merupakan Kelainan Yang Mempengaruhi Kemampuan Bergerak Dan Mempertahankan Keseimbangan Pada Ana1Document18 pagesCerebral Palsy Merupakan Kelainan Yang Mempengaruhi Kemampuan Bergerak Dan Mempertahankan Keseimbangan Pada Ana1egimaruNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- AchalasiaDocument18 pagesAchalasiaegimaruNo ratings yet
- SedentaryDocument17 pagesSedentaryegimaruNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Eating BabyDocument9 pagesEating BabyegimaruNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- GonorrheaDocument3 pagesGonorrheaRini Oyien WulandariNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Acute Hypoglycemia Induces Retinal Cell Death in MouseDocument17 pagesAcute Hypoglycemia Induces Retinal Cell Death in MouseegimaruNo ratings yet
- The Incidence of Decompression Sickness Is RareDocument12 pagesThe Incidence of Decompression Sickness Is RareegimaruNo ratings yet
- New England Journal Medicine: The ofDocument9 pagesNew England Journal Medicine: The ofegimaruNo ratings yet
- MCN Guidelines For The Management of The Severely MalnourishedDocument0 pagesMCN Guidelines For The Management of The Severely MalnourishedegimaruNo ratings yet
- Pro MalariaDocument2 pagesPro MalariaegimaruNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Blood LossDocument2 pagesBlood LossegimaruNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Detailed Study of Malaria: Transmission, Burden and PreventionDocument38 pagesDetailed Study of Malaria: Transmission, Burden and PreventionDayledaniel SorvetoNo ratings yet
- PARASITOLOGY of MALARIA 02Document33 pagesPARASITOLOGY of MALARIA 02selvyNo ratings yet
- Non-chordate Biology Lab GuideDocument184 pagesNon-chordate Biology Lab GuideBheemasenachar KopparNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- ADCO-QUININE DIHYDROCHLORIDE IndicationsDocument4 pagesADCO-QUININE DIHYDROCHLORIDE IndicationsArthas Rhee HermanNo ratings yet
- Plasmodium VivaxDocument4 pagesPlasmodium VivaxSUTHAN100% (1)
- Antimalarial DrugsDocument14 pagesAntimalarial Drugstea jayNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- AsharDocument5 pagesAsharGoodbye ForeverNo ratings yet
- DISEASE - Cholera and MalariaDocument53 pagesDISEASE - Cholera and MalariagizzarNo ratings yet
- Comparative Analysis of Nested PCR and Conventional Microscopic Examination With MalariaDocument59 pagesComparative Analysis of Nested PCR and Conventional Microscopic Examination With MalariaRAHMA TRIYANAYNo ratings yet
- UK malaria treatment guidelines summaryDocument11 pagesUK malaria treatment guidelines summaryikaNo ratings yet
- Antimaleria Drug ModelsDocument33 pagesAntimaleria Drug ModelsMehari AsratNo ratings yet
- Malaria InfectionDocument85 pagesMalaria InfectionspinosumNo ratings yet
- SESSION 8 - Anti-Malaria DrugsDocument48 pagesSESSION 8 - Anti-Malaria DrugsYassboy MsdNo ratings yet
- Malaria Treatment Guideline 2012Document38 pagesMalaria Treatment Guideline 2012van_cristiano100% (3)
- Antimalarial DrugsDocument45 pagesAntimalarial DrugsDeribe BekeleNo ratings yet
- CIF MALARIA - Rev4 As July 4 2016 - LatestDocument3 pagesCIF MALARIA - Rev4 As July 4 2016 - LatestNicholai Cabaddu100% (2)
- A Chilly FeverDocument5 pagesA Chilly FeverChangNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Malaria: Health Education HFT 201 By: Sophia Kol, MDDocument10 pagesMalaria: Health Education HFT 201 By: Sophia Kol, MDTith SeavmeyNo ratings yet
- Parasitology: Prepared By: Charriz A. AmoyanDocument99 pagesParasitology: Prepared By: Charriz A. AmoyanRaphylyn EscalonaNo ratings yet
- Diseases PIDSRDocument25 pagesDiseases PIDSRaringkinking100% (1)
- 17.national Malaria Case Management Guideline 14 January 2022 UnderDocument61 pages17.national Malaria Case Management Guideline 14 January 2022 UnderTizazu BayihNo ratings yet
- Plasmodium Vivax: Laboratory Diagnosis of MalariaDocument4 pagesPlasmodium Vivax: Laboratory Diagnosis of MalariaShaiji ShahidNo ratings yet
- 1 s2.0 S1093326316300055 MainDocument11 pages1 s2.0 S1093326316300055 MainChinar PatelNo ratings yet
- Enterobius Vermicularis: Ciulla Chapter 8 - ParasitologyDocument13 pagesEnterobius Vermicularis: Ciulla Chapter 8 - ParasitologySalve Rachelle BillenaNo ratings yet
- Dipiro 8 ParasitDocument12 pagesDipiro 8 ParasitErik Firman RusdiantoNo ratings yet
- L30 - FPSC Paper Mill Colony C38/1, Gurdwara Road, Siddheshwar LucknowDocument4 pagesL30 - FPSC Paper Mill Colony C38/1, Gurdwara Road, Siddheshwar LucknowShamsuddin ShamsNo ratings yet
- Ebm JurnalDocument198 pagesEbm Jurnalcamelia musaadNo ratings yet
- 002 Chemotherapy of MalariaDocument93 pages002 Chemotherapy of MalariaMatteo FerrariNo ratings yet
- Malaria & Cerebral Malaria: Livia Hanisamurti, S.Ked 71 2018 045Document40 pagesMalaria & Cerebral Malaria: Livia Hanisamurti, S.Ked 71 2018 045Livia HanisamurtiNo ratings yet
- Malaria Epidemiology & PreventionDocument92 pagesMalaria Epidemiology & PreventionritikaritikaNo ratings yet
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (18)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)