Professional Documents
Culture Documents
Photochromism and Piezochromism
Uploaded by
Blake Connolly0 ratings0% found this document useful (0 votes)
750 views6 pagesThe purpose of this experiment was to synthesize 2,2',4,4',5,5'-hexaphenyl-4H,4'H-4,4'-biimidazole and analyse the photochromic and piezochromic properties. The melting point observed differs significantly from literature vale of 187.5-188. 0 C.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe purpose of this experiment was to synthesize 2,2',4,4',5,5'-hexaphenyl-4H,4'H-4,4'-biimidazole and analyse the photochromic and piezochromic properties. The melting point observed differs significantly from literature vale of 187.5-188. 0 C.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
750 views6 pagesPhotochromism and Piezochromism
Uploaded by
Blake ConnollyThe purpose of this experiment was to synthesize 2,2',4,4',5,5'-hexaphenyl-4H,4'H-4,4'-biimidazole and analyse the photochromic and piezochromic properties. The melting point observed differs significantly from literature vale of 187.5-188. 0 C.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 6
Photochromism and Piezochromism
Blake Connolly 27/05/2014
Introduction
The purpose of this experiment was to synthesize 2,2',4,4',5,5'-hexaphenyl-4H,4'H-4,4'-biimidazole
from oxidative coupling of 2,45-triphenyl-1H-imidazole and analyse the photochromic and
piezochromic properties.
Experimental
2,1,5-triphenyl-1H-imidazole (0.97g 0.003mol) was added to ethanol (100ml) follower by potassium
hydroxide pellets (8.09g 0.144mol) and water (5ml). The solution was mixed until a clear. The
solution was cooled to 5
0
C and a mixture of water (250ml), potassium ferricynanide (3.15g,
0.009mol) and ethanol (100ml) was added periodically not allowing the reaction not to exceed 10
0
C.
The precipitate was filtered and washed with 5 x 50ml of water and 10ml of 50% aqueous ethanol.
The product was then thoroughly dried. Yield (0.96g 106%) Observed m.p. 263
0
C (decomp.)
Results & Discussion
The melting point observed differs significantly from literature vale of 187.5-188.5
0
C
[1]
. This is
thought to be due to some unreacted 2,4,52,45-triphenyl-1H-imidazole (m.p. 274-276
0
C)
[2]
. Another
reason could be due to the product being slightly wet. This reason would also explain the yield.The
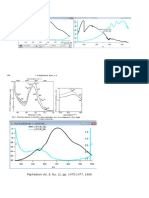
UV-Vis of the product obtained (conc. 2.7mm 400nm - 700nm) peaked at 553nm (0.03A) for the
product before irradiation and 554nm (0.46A) after irradiation. It can be seen clearly that after
irradiation the absorbance of the product drastically increases, it was also noticed that upon irradiation
the solution turned an purple colour. The product when ground turned a dark purple colour and upon
removal and storage in the dark it slowly reverted to a light purple/grey similar to a combination of
both. The coloured species is a reactive intermediate in this case a free radical formed by the cleavage
of the two five-membered ring.
NMR - Dimer 4
1
H
13
C
Chemical
Shift (ppm)
No. of
Hydrogens
Multiplicity
Assignment
Chemical
Shift (ppm)
Assignment
7.26
30
multiplet
Benzene Ring
126.13
Aromatic
4
1
Ethanol
127.08
Aromatic
1.75
2
Water
127.47
Aromatic
1.25
2
Ethanol
127.49
Aromatic
127.57
Aromatic
127.73 Aromatic
127.79 Aromatic
127.97 CN
3
Ph
128.34 Aromatic
129.27 Aromatic
130.23
131.02
132.01
NMR Dimer 3
1
H
13
C
Chemical
Shift (ppm)
No. of
Hydrogens
Multiplicity
Assignment
Chemical
Shift (ppm)
Assignment
7.26
30
multiplet
Benzene Ring
126.13
Aromatic
4
1
Ethanol
127.08
Aromatic
1.75
2
Water
127.47
127.49
Aromatic
Aromatic
1.25
2
Ethanol
127.57
127.73
Aromatic
Aromatic
127.79
128.34
Aromatic
Aromatic
129.27 Aromatic
130.23
131.02
132.01
Questions
1)
The radical formed has the ability to stabilise itself through resonance structures localising the
electrons within the ring.
2)
see attached page.
3)
4)
Photochromic materials are useful in today's society. One use of these are in transition lenses
whereby when the wearer is in the sun they darken and vice-versa. Piezochromic materials change
colour when a force is applied to it. A method is currently being researched for the use of
piezochromic ink in preparation of bank notes as to prevent forgeries.
Conclusion
Reference List
White; Sonnenberg
Journal of the American Chemical Society, 1966 , vol. 88, p. 3825,3828
Teimouri, Abbas; Chermahini, Alireza Najafi
Journal of Molecular Catalysis A: Chemical, 2011 , vol. 346, # 1-2 p. 39 - 45
http://www.google.com/patents/US20070259286
You might also like
- David Belias, Chem 213 Synthetic #3 FFR Experiment 9: Synthesis of A Coumarin Laser DyeDocument9 pagesDavid Belias, Chem 213 Synthetic #3 FFR Experiment 9: Synthesis of A Coumarin Laser Dyedwb530750% (2)
- Chitosan Catalyzed Synthesis of IminesDocument6 pagesChitosan Catalyzed Synthesis of IminesaustingoewertNo ratings yet
- Synthesis of A Coumarin Laser DyeDocument8 pagesSynthesis of A Coumarin Laser Dyesoliman_andro100% (3)
- Nathan Lisbin Synthetic FFR #2 Synthesis of A Coumarin Laser DyeDocument10 pagesNathan Lisbin Synthetic FFR #2 Synthesis of A Coumarin Laser DyeNate LisbinNo ratings yet
- Synthetic FFR 2Document10 pagesSynthetic FFR 2ashNo ratings yet
- Experiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneDocument10 pagesExperiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneColin CheNo ratings yet
- Synthesis of Camphor by The Oxidation of BorneolDocument6 pagesSynthesis of Camphor by The Oxidation of BorneolCyrene MBolañosNo ratings yet
- New Energy Transfer Dyes For DNA SequencingDocument7 pagesNew Energy Transfer Dyes For DNA SequencingFany Monterrubio LozanoNo ratings yet
- Formal Diazo Dye ReportDocument9 pagesFormal Diazo Dye Reportbig504075% (4)
- Temel2010 PDFDocument7 pagesTemel2010 PDFrommy agurto palaciosNo ratings yet
- The Hofmann Rearrangement Using Household Bleach-Synthesis of 3-NitroanilineDocument1 pageThe Hofmann Rearrangement Using Household Bleach-Synthesis of 3-NitroanilineKybernetikumNo ratings yet
- A J B P R: Sian Ournal of Iochemical and Harmaceutical EsearchDocument4 pagesA J B P R: Sian Ournal of Iochemical and Harmaceutical EsearchP RambabuNo ratings yet
- ABx Dendrons Effect of MultiplicityDocument7 pagesABx Dendrons Effect of MultiplicityRasha El-GhazawyNo ratings yet
- 10644959324293111096Document6 pages10644959324293111096Danesh AzNo ratings yet
- An Efficient One-Pot Strategies For The Synthesis of (1,3) Oxazine DerivativesDocument6 pagesAn Efficient One-Pot Strategies For The Synthesis of (1,3) Oxazine DerivativesANBU DINESHNo ratings yet
- Treatment of Dye Wastewater by Using Photo-Catalytic Oxidation With SonicationDocument6 pagesTreatment of Dye Wastewater by Using Photo-Catalytic Oxidation With SonicationJorn DoeNo ratings yet
- An Optimal Procedure For Ammoniacal Nitrogen Analysis in Natural Waters Using Indophenol Blue MethodDocument10 pagesAn Optimal Procedure For Ammoniacal Nitrogen Analysis in Natural Waters Using Indophenol Blue MethodAndre PNo ratings yet
- (Bmim) OH222Document10 pages(Bmim) OH222majidNo ratings yet
- Relative Acidity Measurement of Bronsted Acid Functional Ionic Liquids by UV-spectrosDocument8 pagesRelative Acidity Measurement of Bronsted Acid Functional Ionic Liquids by UV-spectrosela arasuNo ratings yet
- 4 +Proline-Catalyzed+AsymmetriDocument8 pages4 +Proline-Catalyzed+Asymmetricmc107No ratings yet
- Era Melania - PKU 18 - 18030194085 - Jurnal Praktikum Penentuan Jenis Asam Amino Dalam SampelDocument8 pagesEra Melania - PKU 18 - 18030194085 - Jurnal Praktikum Penentuan Jenis Asam Amino Dalam SampelEra MelaniaNo ratings yet
- The Fischer Esterification of BenzocaineDocument5 pagesThe Fischer Esterification of BenzocaineMikeNo ratings yet
- Exp. # 8 Polymers, Polymerization, and AnalysisDocument14 pagesExp. # 8 Polymers, Polymerization, and AnalysisAlyssa FerenceNo ratings yet
- Reductive Methylation of Primary and Secondary Amines and Amino Acids by Aqueous Formaldehyde and ZincDocument3 pagesReductive Methylation of Primary and Secondary Amines and Amino Acids by Aqueous Formaldehyde and Zincjavasolo100% (1)
- Green Condensation Reaction of AromaticDocument11 pagesGreen Condensation Reaction of AromaticJosé Guadalupe García EstradaNo ratings yet
- Synthesis of A Diazo DyeDocument8 pagesSynthesis of A Diazo DyeLucas Man100% (1)
- Interference With Ammonium Determination by The Indophenol-Type Reaction of Salicylate and DichloroisocyanurateDocument2 pagesInterference With Ammonium Determination by The Indophenol-Type Reaction of Salicylate and DichloroisocyanurateDiễn Đàn Hóa HọcNo ratings yet
- The Hoftnann Rearrangement Using Bleach: Synthesis of 3-NitroanilineDocument1 pageThe Hoftnann Rearrangement Using Bleach: Synthesis of 3-Nitroanilineatomosco100% (1)
- Observation of Anthracene Excimer Fluorescence at Very Low Concentrations Utilizing Dendritic StructuresDocument3 pagesObservation of Anthracene Excimer Fluorescence at Very Low Concentrations Utilizing Dendritic StructuresSreedevi KrishnakumarNo ratings yet
- Dinoflagellate Luciferin Is Structurally Related To ChlorophyllDocument4 pagesDinoflagellate Luciferin Is Structurally Related To ChlorophylljavierNo ratings yet
- Synthesis of 1 Phenylazo 2 NaphtholDocument8 pagesSynthesis of 1 Phenylazo 2 NaphtholChris Zayn0% (1)
- Bromination of AcetanilideDocument7 pagesBromination of Acetanilideaustingoewert93% (15)
- The Synthesis and Pharmacology of Ephedrine Analogues PDFDocument123 pagesThe Synthesis and Pharmacology of Ephedrine Analogues PDFStephan Gregor100% (1)
- Moses 1978Document6 pagesMoses 1978Puku KunNo ratings yet
- Effects of Ozonation vs. Chlorination Water Treatment Operations On Natural Organic Matter FractionsDocument16 pagesEffects of Ozonation vs. Chlorination Water Treatment Operations On Natural Organic Matter FractionsdimateaNo ratings yet
- Solvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesDocument6 pagesSolvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesbaskhemNo ratings yet
- Colorants With Mixt Naphthoquinone-Azomethine Cromophore-Synthesis, Characterization and in Vitro Toxicity AnalysisDocument14 pagesColorants With Mixt Naphthoquinone-Azomethine Cromophore-Synthesis, Characterization and in Vitro Toxicity AnalysisRoxana IonescuNo ratings yet
- Seminar Ankita 2Document17 pagesSeminar Ankita 2Ankita RoyNo ratings yet
- Synthesis of New Metal-Free and Metal-Containing Phthalocyanines With Tertiary or Quaternary Aminoethyl SubstituentsDocument7 pagesSynthesis of New Metal-Free and Metal-Containing Phthalocyanines With Tertiary or Quaternary Aminoethyl SubstituentsFrancisco Batista Do NascimentoNo ratings yet
- Demethylation With LiCl-DMF (JMolCatA-Chemical2007)Document8 pagesDemethylation With LiCl-DMF (JMolCatA-Chemical2007)Archawin_mooNo ratings yet
- Organic Chemistry Lab ReportDocument12 pagesOrganic Chemistry Lab Reportcyc5326100% (1)
- SintezaDocument2 pagesSintezaljubicasta_314430557No ratings yet
- Spectrophotometric Determination of Chromium in Water, and Pharmaceutical Samples Using 1-NaphtholDocument10 pagesSpectrophotometric Determination of Chromium in Water, and Pharmaceutical Samples Using 1-NaphtholJoanne YapNo ratings yet
- Molecules: New 3H-Indole Synthesis by Fischer's Method. Part IDocument8 pagesMolecules: New 3H-Indole Synthesis by Fischer's Method. Part ILEONETTILENCINANo ratings yet
- Art 3A10.1007 2Fs00289 016 1670 yDocument12 pagesArt 3A10.1007 2Fs00289 016 1670 yilham ramNo ratings yet
- Synthesis and Characterization of Some Novel Coumarin Based Pyrazoles, Isoxazole and Pyrimidyl DerivativesDocument6 pagesSynthesis and Characterization of Some Novel Coumarin Based Pyrazoles, Isoxazole and Pyrimidyl DerivativesNalla Umapathi ReddyNo ratings yet
- Physical Data of Compounds Used in Organic Chemistry Labs: IV.1 Concentrated Acids and BasesDocument15 pagesPhysical Data of Compounds Used in Organic Chemistry Labs: IV.1 Concentrated Acids and BasesAlodia Eunicia Orata CastilloNo ratings yet
- The Heterogenation of Melamine and Its Catalytic Activity: Applied Catalysis A: GeneralDocument7 pagesThe Heterogenation of Melamine and Its Catalytic Activity: Applied Catalysis A: GeneralJimmy NelsonNo ratings yet
- Ma 8012477Document7 pagesMa 8012477Friska SiamiNo ratings yet
- Penjelasan Fenton Dan Foto FentonDocument8 pagesPenjelasan Fenton Dan Foto FentonNurillahi Febria LeswanaNo ratings yet
- Benzocaine Syntheisi Via Fischer EsterificationDocument7 pagesBenzocaine Syntheisi Via Fischer EsterificationXiang Yu100% (7)
- Phytochemical Analysis, Isolation and Identification of Flavan-3ol From Syrian Pinus HalepensisDocument7 pagesPhytochemical Analysis, Isolation and Identification of Flavan-3ol From Syrian Pinus HalepensisリファイNo ratings yet
- Bromination of AcetanilideDocument7 pagesBromination of AcetanilideaustingoewertNo ratings yet
- Figure 1 Synthesis of DilantinDocument9 pagesFigure 1 Synthesis of Dilantinstepkim92No ratings yet
- Indigo DecolorationDocument9 pagesIndigo Decolorationmazahir razaNo ratings yet
- Terje MahanDocument15 pagesTerje MahanErikaNo ratings yet
- Ion Exchange Resins and Adsorbents in Chemical Processing: Second EditionFrom EverandIon Exchange Resins and Adsorbents in Chemical Processing: Second EditionRating: 5 out of 5 stars5/5 (1)
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974From EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannNo ratings yet
- Iridium Complexes in Organic SynthesisFrom EverandIridium Complexes in Organic SynthesisLuis A. OroNo ratings yet
- Synthesis of 2-methyl-4-selenoquinazolone, 2-phenylbenzoselenazole, and its derivativesFrom EverandSynthesis of 2-methyl-4-selenoquinazolone, 2-phenylbenzoselenazole, and its derivativesNo ratings yet
- Mid-Term Exam RevisionDocument31 pagesMid-Term Exam RevisionBlake ConnollyNo ratings yet
- Information and Guidelines For The NMR AssignmentDocument4 pagesInformation and Guidelines For The NMR AssignmentBlake ConnollyNo ratings yet
- Dbms Responsive Ness Integrati On Scalabili Ty Ease of Setup Cost Final Hardoop (Spark/H Ive) Mongod BDocument2 pagesDbms Responsive Ness Integrati On Scalabili Ty Ease of Setup Cost Final Hardoop (Spark/H Ive) Mongod BBlake ConnollyNo ratings yet
- Pdyhedron Vol. 9, No. 12, Pp. 1475-1477, 1990Document2 pagesPdyhedron Vol. 9, No. 12, Pp. 1475-1477, 1990Blake ConnollyNo ratings yet
- Waffle RecipeDocument1 pageWaffle RecipeBlake ConnollyNo ratings yet
- Christ, Be Our Light! Shine in Our Hearts. Shine Through The Darkness. Christ, Be Our Light! Shine in Your Church Gathered TodayDocument1 pageChrist, Be Our Light! Shine in Our Hearts. Shine Through The Darkness. Christ, Be Our Light! Shine in Your Church Gathered TodayBlake ConnollyNo ratings yet
- Felix MendelssohnDocument1 pageFelix MendelssohnBlake ConnollyNo ratings yet
- Bella Bartok: Click To Hear Bartok's MusicDocument11 pagesBella Bartok: Click To Hear Bartok's MusicBlake ConnollyNo ratings yet
- Ethical ReviewDocument2 pagesEthical ReviewBlake ConnollyNo ratings yet
- UnderTheSea (Elise)Document11 pagesUnderTheSea (Elise)Blake ConnollyNo ratings yet
- MRCellophaneDocument8 pagesMRCellophaneBlake ConnollyNo ratings yet
- DefinitionsDocument1 pageDefinitionsBlake ConnollyNo ratings yet
- MRCellophaneDocument8 pagesMRCellophaneBlake ConnollyNo ratings yet
- Spring Break ChemistryDocument2 pagesSpring Break Chemistryapi-213793181No ratings yet
- Optician's Guide (A Manual For Opticians)Document109 pagesOptician's Guide (A Manual For Opticians)norma paulina carcausto lipaNo ratings yet
- Chemistry Form 4 (Manufactured Substances in Industries)Document24 pagesChemistry Form 4 (Manufactured Substances in Industries)Fariezuan HamidNo ratings yet
- Photochromic Lenses HandoutDocument2 pagesPhotochromic Lenses HandoutLewis Clark100% (2)
- Prime Lens Brochure Pages Set 10 - Lens (Optics) - Lenses PDFDocument15 pagesPrime Lens Brochure Pages Set 10 - Lens (Optics) - Lenses PDFDipesh ChoudharyNo ratings yet
- 262 - PDFsam - DLP TEXTBOOK Chemistry FORM 4 PDFDocument25 pages262 - PDFsam - DLP TEXTBOOK Chemistry FORM 4 PDFnaseem wanNo ratings yet
- Serengeti 2013 Catalogue UKDocument60 pagesSerengeti 2013 Catalogue UKJesus Jarabo Sevilla100% (1)
- Photochromism and PiezochromismDocument6 pagesPhotochromism and PiezochromismBlake ConnollyNo ratings yet
- BBB 2009Document88 pagesBBB 2009Endre MiklosNo ratings yet
- Bollé Solar 2012 UKDocument60 pagesBollé Solar 2012 UKGojko GošovićNo ratings yet
- Ilt Product Catalogue 2015Document29 pagesIlt Product Catalogue 2015Alex Hoong LimNo ratings yet
- Photochromatic LensDocument38 pagesPhotochromatic LensSrinivas DonNo ratings yet
- Final For V.M 2Document21 pagesFinal For V.M 2jatans_1No ratings yet
- Opthalmic OpticsDocument32 pagesOpthalmic OpticsOptical SystemNo ratings yet
- E16 Composite MaterialsDocument36 pagesE16 Composite MaterialsNoor Fazlyana Sabri0% (1)
- Composite Materials and Its ImportanceDocument12 pagesComposite Materials and Its ImportanceIVAN TIONG WEI JUN MoeNo ratings yet
- Revision Sawfly Photochromic ReviewDocument2 pagesRevision Sawfly Photochromic ReviewTwobirds Flying PublicationsNo ratings yet
- Organic Photochromic and Thermochromic Compounds Photochromic Families by John C. Crano, Robert J. GuglielmettiDocument401 pagesOrganic Photochromic and Thermochromic Compounds Photochromic Families by John C. Crano, Robert J. GuglielmettiSiddhesh Umesh MestryNo ratings yet
- ZEISS RX Catalogue 2019 Online UK 4-3-19Document181 pagesZEISS RX Catalogue 2019 Online UK 4-3-19Petros Moutsopoulos0% (1)
- 2010 Serengeti Sunglasses CatalogDocument25 pages2010 Serengeti Sunglasses Catalogbrdrider77No ratings yet
- Limar Eyewear 2015Document21 pagesLimar Eyewear 2015Lukasz BorowiczNo ratings yet
- Reversible SunglassesDocument3 pagesReversible SunglassesARCUSNo ratings yet
- Chemistry Folio Form 4 2013Document17 pagesChemistry Folio Form 4 2013Megat AshmannNo ratings yet
- Absorptive - Lenses 5 1Document49 pagesAbsorptive - Lenses 5 1M. Sohaib Ali KhanNo ratings yet
- 2022 Dispensing Tips TransitionsDocument2 pages2022 Dispensing Tips TransitionsSayoki ghosgNo ratings yet