Professional Documents
Culture Documents
Acids and Bases Notes
Uploaded by
cgao30Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acids and Bases Notes
Uploaded by
cgao30Copyright:
Available Formats
Crystal Gao
IB Chemistry 2013
1 | P a g e
Acids and Bases notes
(Topics 8 & 18)
8.1 Theories of acids and bases (SL)
8.1.1 Define acids and bases according to the Bronsted-Lowry and Lewis theories (1)
Bronsted-Lowry:
Acid proton donor; involves transfer of H
+
ion one species to another
e.g. HCl
(g)
+ H
2
O
(l)
H
3
O
+
(aq)
+ Cl
-
(aq)
Base proton acceptor; involves acceptance of H
+
ion from another species
e.g. NH
3(aq)
+ H
2
O
(l)
NH
4
+
(aq)
+ OH
-
(aq)
Lewis:
Hypothesised that acid-base behavior is not tied to water or restricted to protons
Acid electron-pair acceptor
Base electron-pair donor
Donation of an e
-
pair by a Lewis base to a Lewis acid creates a covalent bond b/w species
Covalent bond known as a dative/coordinate bond because the two share e
-
come from the
same species (represented by arrow with a head [] from e
-
donor to e
-
acceptor)
Lewis acids are often electron deficient, hence very reactive towards e
-
pair donor (e.g. BF
3
)
e.g.BF
3
+ NH
3
BF
3
NH
3
Lewis acid + Lewis base Lewis acid-base complex
Arrhenius:
Acidic solution caused by H
+
(aq)
(released into solution via dissociation)
Basic solution caused by OH
-
(aq)
Crystal Gao
IB Chemistry 2013
2 | P a g e
8.1.2 Deduce whether or not a species could act as a Bronsted-Lowry and/or a Lewis acid/base (3)
Amphiprotic:
Substances that can act both as a base and an acid
E.g. water (H
2
O), hydrogen carbonate ion (HCO
3
-
), hydrogen sulfate ion (HSO
4
-
)
e.g. HCl
(aq)
+ H
2
O
(l)
H
3
O
+
(aq)
+ Cl
-
(aq)
NH
3(aq)
+ H
2
O
(l)
NH
4
+
(aq)
+ OH
-
(aq)
8.1.3 Deduce the formula of the conjugate acid (or base) of any Bronsted-Lowry base/acid (3)
Bronsted Lowry theory states that a base, after receiving a proton, has the potential to react
as an acid (and vice versa)
i.e. acid-base reactions are reversible
e.g. CH
3
COO
-
(aq)
+ H
2
O
(l)
Cl
-
(aq)
+ H
3
O
+
(aq)
Acid + base conjugate base + conjugate acid
acid
base
conjugate base
conjugate acid
H
2
SO
4
+ CH
3
COOH HSO
4
-
+ CH
3
COOH
2
+
CH
3
COOH + NH
3
CH
3
COO- + NH
4
+
HCl + H
2
O Cl- + H
3
O
+
proton donor
proton
acceptor
proton
acceptor
proton donor
Example: Ammonia + hydrogen chloride
In the reaction between ammonia gas and hydrogen chloride the hydrogen chloride transfers a hydrogen ion to
the ammonia making an ammonium ion. In this reaction the ammonia is behaving as a Brnsted Lowry base by
accepting a proton (hydrogen ion). The hydrogen chloride is a Brnsted-Lowry acid for providing (donating) that
proton (hydrogen ion)
HCl + NH
3
NH
4
Cl
Crystal Gao
IB Chemistry 2013
3 | P a g e
8.2 Properties of acids and bases
8.2.1 Outline the characteristic properties of acids and bases in aqueous solutions (2)
Acids:
Tastes sour (usually
Turns blue litmus red
Corrosive
Molecular in structure
Conducts electricity in aqueous solution
Reactions
1. Acids react with bases to form salt and water (neutralisation). The base may be a
metal oxide or a metal hydroxide.
e.g. HCl
(aq)
+ NH
3(aq)
NH
4
Cl
(aq)
2. Acids react with metal carbonates forming a salt, carbon dioxide and water
(neutralisation)
e.g. 2HCl
(aq)
+ CaCO
3(s)
CaCl
2(aq)
+ CO
2(g)
+ H
2
O
(l)
3. Acids react with active metals to form salt and hydrogen (this is not actually a
neutralisation reaction although the acid does get used up)
e.g. 2HCl
(aq)
+ Mg
(s)
MgCl
2(aq)
+ H
2(g)
4. Reacts with metal hydroxides to produce salt and water
e.g. 2HCl
(aq)
+ Mg(OH)
2(s)
MgCl
2(aq)
+ 2H
2
O
(l)
5. Reacts with metal oxides to produce a salt and water
e.g. 2HCl
(aq)
+ MgO
(s)
MgCl
2(aq)
+ H
2
O
(l)
6. Reacts with metal hydrogen carbonates to produce a salt, water and carbon dioxide
e.g. 2HCl
(aq)
+ Mg(HCO
3
)
2(s)
MgCl
2(aq)
+ 2H
2
O
(l)
+ 2CO
2(g)
Only H
+
ions reacting; Cl
-
ions remain in solution as spectator ions
Crystal Gao
IB Chemistry 2013
4 | P a g e
Bases:
Taste bitter
Turn red litmus blue
Feel slippery (strong bases convert skin oil to soap)
Conducts electricity in aqueous solution
Carbonates are basic
Reactions:
Same as list for acids
1. Ammonia reacts with acids to produce an ammonium salt
e.g. NH
3(aq)
+ HCl
(aq)
NH
4
Cl
(aq)
8.3 Strong and weak acids and bases
8.3.1 Distinguish between strong and weak acids and bases in terms of the extent of dissociation,
reaction with water and electrical conductivity (2)
Strong acids Strong acids tend to dissociate fully (or nearly 100%) in water
e.g. HCl
(aq)
+ H
2
O
(l)
H
3
O
+
(aq)
+ Cl
-
(aq)
Weak acids only dissociate partially in water (<1%)
e.g. CH
3
COOH
(aq)
+ H
2
O
(l)
H
3
O
+
(aq)
+ CH
3
COO
-
(aq)
8.3.2 State whether a given acid or base is strong or weak (1)
ACID BASE
Strong Weak Strong Weak
HCl CH
3
OOH Group 1
hydroxides
NH
3
HNO
3
H
2
CO
3
Ba(OH)
2
C
2
H
4
NH
3
H
2
SO
4
- - -
Crystal Gao
IB Chemistry 2013
5 | P a g e
8.3.3 Distinguish between strong and weak acids and bases, and determine the relative
strengths of acids and bases, using experimental data (2)
Energy of neutralisation (i.e. amount of acid/base required to neutralise it)
Conductivity
Rates of reaction
pH measurement
8.4 The pH scale
8.4.1 Distinguish between aqueous solutions that are acidic, neutral or alkaline using the pH
scale (2)
pH > 7 alkaline
pH = 7 neutral
pH < 7 acidic
8.4.2 Identify which of two or more aqueous solutions is more acidic/alkaline using pH values (2)
The greater the pH, the more alkaline
The smaller the pH, the more acidic
8.4.3 State that each change of one pH units = a 10-fold change in [H
+
(aq)
] (1)
Each change of one pH unit = 10-fold change in [H
+
(aq)
]
E.g. If a beaker of HCl has a [H
+
] of 1.0M and pH of 1, then when the pH = 2, the [H
+
] = 0.1M
8.4.4 Deduce changes in [H
+
(aq)
] when the pH of a solution changes by more than one pH unit (3)
pH = -log
10
[H
+
]
pOH = -log
10
[OH
-
]
pH = 14 pOH (and vice versa)
Crystal Gao
IB Chemistry 2013
6 | P a g e
18.1 Calculations involving acids and bases (HL)
18.1.1 State the expression for the ionic product constant of water (K
w
) (1)
Equilibrium expression for the self-ionisation of water at 25C
K
w
= [H
3
O
+
][OH
-
] = 1.00 x 10
-14
mol
2
dm
-6
at 25C
18.1.2 Deduce [H
+
(aq)
] and [OH
-
(aq)
] for water at different temperatures given K
w
values (3)
H
2
O
(l)
+ H
2
O
(l)
H
3
O
+
(aq)
+ OH
-
(aq)
H = +57kJ mol
-1
Endothermic, therefore energy is required to break the bonds
According to Le Chateliers principle, increase in temperature favours the forward reaction
i.e. equilibrium shifts towards the right and K
w
value increases
As ratio of [H
+
] must remain equal to [OH
-
] in pure water, then if we know K
w
, we can easily
calculate concentrations of [H
+
] and [OH
-
], and hence values of pH and pOH
1. K
w
= [H
3
O
+
][OH
-
]
2. [H
3
O
+
] = [OH
-
]
3. K
w
= [H
3
O
+
]
2
4. [H
3
O
+
+ = K
w
= [OH
-
]
18.1.3 Solve problems involving [H
+
(aq)
], [OH
-
(aq)
], pH and pOH (3)
pH:
pH = -log
10
[H
+
(aq)
]
[H
+
(aq)
] = 10
-pH
pOH:
pOH = -log
10
[OH
-
(aq)
]
[OH
-
(aq)
] = K
w
/ [H
+
(aq)
]
Crystal Gao
IB Chemistry 2013
7 | P a g e
pK
w
:
pK
w
= -log
10
K
w
pK
w
= pH + pOH = 14
Calculation of the pH of a weak base:
1. Find the [OH-] from the pKb value (in the same way as for weak acid type calculations)
2. From the [OH-] find the pOH
3. Find the pH from: pOH + pH = 14
Example:
Stage 1:
pH of 0.5 M phenol (pKa = 9.83)
pKa = 9.83 therefore pKb = 14 9.83
pKb = 4.07
Kb = 10
-4.07
= 8.51 x 10
-5
Kb x [phenol] = [X+][OH-]
[OH-+ = (Kb x *phenol+)
[OH-+ = (8.51 x 10
-5
x 0.5)
[OH-] = 6.52 x 10
-3
Stage 2:
pOH = -log
10
[OH
-
]
pOH = 2.19
Stage 3:
pH = 14 2.19
pH = 11.81
18.1.4 State the equation for the reaction of any weak acid or weak base with water and hence
deduce the expressions for K
a
and K
b
(1)
Weak acid with water:
HA
(aq)
+ H
2
O
(l)
A
-
(aq)
+ H
3
O
+
(aq)
K
a
= [A
-
][H
3
O
+
]
[HA]
Weak base with water:
B
(aq)
+ H
2
O
(l)
HB
+
(aq)
+ OH
-
(aq)
K
b
= [HB
+
][OH
-
]
[B]
Crystal Gao
IB Chemistry 2013
8 | P a g e
18.1.5 Solve problems involving solution of weak acids and bases using the expressions (3):
Note that for strong acids and bases [H
+
] or [OH
-
] are directly related to the concentration of
the acid/base
Therefore doubling the concentration of the acid will double [H
+
] and halve [OH
-
] (and the
reverse is true for bases).
HA
(aq)
H
+
(aq)
+ A
-
(aq)
B
(aq)
+ H
2
O
(l)
BH
+
+ OH
-
(aq)
1. K
a
= ( [H+][A-] / [HA] )
2. K
b
= ( [BH+][OH-] / [B] )
3. pK
a
= -log(K
a
)
4. pK
b
= -log(K
b
)
5. K
a
x K
b
= K
w
(i.e. they equal 1 x 10
-14
at 25C)
6. pK
a
+ pK
b
= pK
w
(ie 14 at 25C)
For any weak acid and its conjugate base: K
a
x K
b
= K
w
18.1.6 Identify the relative strengths of acids and bases using values of K
w
, K
b,
pK
a
and pK
b
(2)
Summary strong acid weak acid strong base weak base
Ka very large Large very low low
pKa very low 0 - 2 2-7 high 13-14 7-12
Crystal Gao
IB Chemistry 2013
9 | P a g e
18.2 Buffer solutions
18.2.1 Describe the composition of a buffer solution and explain its action (3)
Buffer solutions resist changes in pH when a small amount of an acid or a base is added
Acidic buffer:
HA
(aq)
+ H
2
O
(l)
A
-
(aq)
+ H
3
O
+
(aq)
Weak acid conjugate base
Equilibrium lies far to the left
Larger proportion of reactants than products
MOH
(aq)
+ HA
(aq)
H
2
O
(l)
+ MA
(aq)
Strong base + weak acid salt
The salt then dissociates completely:
MA
(aq)
M
+
(aq)
+ A
-
(aq)
Addition of salt to weak acid produces a mixture that has equally high proportions of HA
(aq)
and A
-
(aq)
A
-
(aq)
+ H
+
(aq)
HA
(aq)
When a small amount of acid is added to the buffer, the pH initially decreases
Conjugate base ions, A
-
, react with the added H
+
ions, causing pH to increase back to original
OH
-
(aq)
+ H
+
(aq)
H
2
O
(l)
When a small amount of base is added, the pH initially increases as H
+
(aq)
is removed by
reaction with OH
-
(aq)
Weak acid dissociates to increase [H
+
(aq)
] and hence decrease pH to original level
Buffer solution:
B
(aq)
+ H
2
O
(l)
BH
+
(aq)
+ OH
-
(aq)
Weak base conjugate acid
HX
(aq)
+ B
(aq)
BHX
(aq)
Strong acid + weak base salt
Crystal Gao
IB Chemistry 2013
10 | P a g e
Summary of composition and behaviour of buffer solutions
Buffer
solution
Made up of When small amount of acid
added
When small amount of
base added
Acidic Weak acid, e.g. ethanoic
acid, CH
3
COOH
pH initially decreases
Conjugate base reacts
A
-
(aq)
+ H
+
(aq)
HA
(aq)
pH increases as H
+
reacts
pH initially decreases
H
+
(aq)
+ OH
-
(aq)
H
2
O
(l)
Weak acid dissociates
HA
(aq)
H
+
(aq)
+ A
-
(aq)
pH decreases as HA
dissociates
Basic Weak base, e.g. ammonia,
NH
3
Weak base reacts
B
(aq)
+ H
+
(aq)
BH
+
(aq)
pH increases as H
+
reacts
pH initially increases
Conjugate acid
dissociates
BH
+
(aq)
H
+
(aq)
+ B
(aq)
pH decreases as
conjugate acid
dissociates
18.2.2 Solve problems involving the composition and pH of a specified buffer system (3)
Calculation of buffer pH
The pH of a buffer solution can be found from the expression for Ka
K
a
= [H+][A-] / [HA]
this can be rearranged to
[H
+
] = K
a
x [HA]/[A
-
]
or
pH = pKa - log [HA]/[A
-
]
Due to the presence of salt in the buffer solution, there is a large reservoir of A
-
(aq)
present
that pushes K
w
to the left
HA
(aq)
+ H
2
O
(l)
A
-
(aq)
+ H
3
O
+
(aq)
Can assume that [HA] at equilibrium is essentially equal to the initial concentration of HA i.e.
concentration of weak acid in the buffer solution
[HA]
eq
[HA]
I
= [acid]
Similarly
[A
-
]
eq
[A
-
]
I
= [salt]
Crystal Gao
IB Chemistry 2013
11 | P a g e
[H
3
O
+
] = K
a
x [acid]
[salt]
pH = pK
a
+ log
10
[salt]
[acid]
pOH = pK
b
+ log
10
[salt]
[base]
Therefore, [H
3
O
+
] = K
a
and pH = pK
a
18.3 Salt hydrolysis
18.3.1 Deduce whether salts form acidic, alkaline or neutral aqueous solutions (3)
Salts involving ions with a high charge density
Solvation
Ionic compounds dissociate 100% into ions in solution. These ions become solvated by the
water molecules (the water molecules bond to the ions - this is one of the driving forces
behind dissolution). The polar water molecules use the lone pairs on the oxygen of the
water to coordinate to the positive metal ion. The ions are then enclosed by a 'cage' of
water molecules usually in an octahedral arrangement.
Octahedral arrangement of water molecules around a
positive ion (in this case a 3+ ion)
Crystal Gao
IB Chemistry 2013
12 | P a g e
Charge density
This means the charge to size ratio of the ion.
charge density = ionic charge/ionic size
When the ion has a charge of 3+ or when it is very small this charge to size ratio is enough
to polarise the water molecules surrounding the ion in solution. This results in a weakening
of the O-H bonds within the water molecules allowing hydrogen ions to be released into
the solution. Hence the solutions are acidic.
This effect is typified in aluminium salts (the aluminium ion has a charge of 3+) which are
very acidic in solution
The aluminium hexaaqua ion
Aluminium ions are surrounded by six water molecules in an octahedral arrangement. This
is called the aluminium hexaaqua ion. The high charge density of the aluminium ion
polarises the water molecules and hydrogen ions are released into solution. The solution is
so acidic that it releases carbon dioxide from sodium carbonate (this reaction is used in
some fire extinguishers to produce foam in conjunction with detergent)
[Al(H
2
O)
6
]
3+
[Al(OH)(H
2
O)
5
]
2+
+ H
+
[Al(OH)(H
2
O)
5
]
2+
[Al(OH)
2
(H
2
O)
4
]
+
+ H
+
Transition metals
As the transition metals have variable oxidation states the ions that are formed with high
charges (high oxidation state) also produce acidic solutions. A good examle of this is the
Iron III ion. Salts such as iron III sulphate are acidic in solution.
[Fe(H
2
O)
6
]
3+
[Fe(OH)(H
2
O)
5
]
2+
+ H
+
Crystal Gao
IB Chemistry 2013
13 | P a g e
Summary of salt hydrolysis
pH of solution Neutral Acidic Basic Depends on K
a
and
K
b
Combination of
acid and base to
make salt
Strong acid +
strong base
Strong acid + weak
base
OR
Strong acid anion +
highly charged
metal cation
Weak acid + strong
base
Weak acid + weak
base
Examples NaCl
KNO
3
NH
4
NO
3
CH
3
NHCl
Al(NO
3
)
3
FeCl
3
CH
3
COOK
HCOONa
CH
3
COONH
4
NH
4
CN
18.5 Indicators
18.5.1 Describe qualitatively the action of an acid-base indicator (2)
Indicators show when a neutralisation reaction is complete in most acid-base titrations
Acid-base indicators are usually weak acids, the dissociation of which can be represented as
HIn
(aq)
H
+
(aq)
+ In
-
(aq)
Colour 1 colour 2
When [H
+
] increases, equilibrium shifts to the left
When [H
+
] decreases, equilibrium shifts to the right
Point at which colour change occurs is called the endpoint
18.5.2 State and explain how the pH range of an acid-base indicator relates to its pK
a
value (3)
pH at which indicator will change colour can be calculated using the pK
a
of the indicator,
otherwise known as pK
in
KIn
(aq)
H
+
(aq)
+ In
-
(aq)
K
in
= [H
+
][In
-
]
[HIn]
-log
10
K
in
= -log
10
[H
+
][In
-
]
[HIn]
Crystal Gao
IB Chemistry 2013
14 | P a g e
pK
a
= pH log
10
[In
-
]
[HIn]
OR
pH = pK
in
+ log
10
[In]
[HIn]
The pH at the endpoint of the indicator is equal to the pK
a
of the indicator, pK
in
18.5.3 Identify an appropriate indicator for a titration, given the equivalence point of the
titration and the pH range of the indicator (3)
Indicator pKa Useful range
Methyl orange 3.7 3.1 - 4.4
Bromophenol blue 4.0 3.0 - 4.6
Methyl red 5.1 4.2 - 6.3
Bromothymol blue 7.0 6.0 - 7.6
Phenol red 7.9 6.8 - 8.4
Phenolphthlein 9.3 8.2 - 10.0
No indicators suitable for indicating the equivalence point for a titration of a weak acid with
a weak base
Equivalence point is where the ratio of moles of acid to moles of base is equal to the
stoichiometric ratio neither reactant is in excess
Endpoint is point where indicator changes colour very close to the equivalence point,
usually 1 or 2 drops after
.
Crystal Gao
IB Chemistry 2013
15 | P a g e
18.4 Acid-base titrations
18.4.1 Sketch the general shapes of graphs of pH against volume for titrations involving strong
and weak acids and bases, and explain their important features (3)
General
Type
Example
Typical Titration
Curve
Features of Curve
Strong acid
& Strong
Base
HCl added to NaOH
Curve begins at high pH typical of strong base
and ends at low pH typical of strong acid.
There is a large rapid change in pH near the
equivalence point (pH =7).
Strong
base &
strong acid
NaOH added to HCl
Curve begins at low pH typical of strong acid,
and ends at high pH typical of strong base.
There is a large rapid change in pH near the
equivalence point (pH=7).
Weak acid
& Strong
base
NaOH added to
ethanoic acid
(CH
3
COOH)
Curve begins at a higher acidic pH and ends at
high basic pH. The pH change at the
equivalence point (pH > 7) is not so great.
Strong acid
& Weak
base
Ammonia (NH
3
)
added to HCl
Curve begins at low pH and ends at a less
high basic pH. The pH change at the
equivalence point (pH < 7) is similar to that
for Strong base & Weak acid.
Weak acid
& Weak
base
Ammonia (NH
3
)
added to ethanoic
acid (CH
3
COOH)
Curve begins at higher acidic pH and ends at
low basic pH. There is not a great pH change
at the equivalence point (pH ~ 7) making this
a very difficult titration to perform.
You might also like
- IB CHEM SL HL Acids and Bases Note CardsDocument61 pagesIB CHEM SL HL Acids and Bases Note Cards陳定均No ratings yet
- Stoichiometry ProblemsDocument4 pagesStoichiometry Problemsphilippeprean0% (1)
- IB Acids and BasesDocument45 pagesIB Acids and BasesAhmad Hajj AliNo ratings yet
- 5.2 (152 Marks) : 1. (1 Mark)Document42 pages5.2 (152 Marks) : 1. (1 Mark)Semwezi EnockNo ratings yet
- 7 Acids and BasesDocument18 pages7 Acids and BasesWong Wai LunNo ratings yet
- Ap Chemistry Unit 5 Notes - ThermodynamicsDocument6 pagesAp Chemistry Unit 5 Notes - Thermodynamicsapi-336799605No ratings yet
- A1 Ch19studyguideDocument3 pagesA1 Ch19studyguideJana Aldour100% (2)
- Periodicity NotesDocument5 pagesPeriodicity Notescgao30No ratings yet
- AP Chemistry - Iodine Clock Reaction Lab ReportDocument4 pagesAP Chemistry - Iodine Clock Reaction Lab ReportJustin MorrowNo ratings yet
- AP Chemistry Chapter 20 Electrochemistry Practice Free Response 1Document2 pagesAP Chemistry Chapter 20 Electrochemistry Practice Free Response 1phuonglehuuyenNo ratings yet
- AP Chemistry Study GuideDocument11 pagesAP Chemistry Study Guidesarah2941No ratings yet
- AP Chem Test - Chapter 10,11,13 - Gases, Solutions, Solids, Liquids, Inter Forces (2010-2011)Document12 pagesAP Chem Test - Chapter 10,11,13 - Gases, Solutions, Solids, Liquids, Inter Forces (2010-2011)dlloyd63050% (2)
- Topic 1 Quantitative Chemistry V3Document34 pagesTopic 1 Quantitative Chemistry V3Aruba Dhaduk100% (2)
- Chemistry - MCQDocument30 pagesChemistry - MCQjoydeep_d32320% (1)
- 09 - Flinn - Stoichiometric Ratio of A ReactionDocument8 pages09 - Flinn - Stoichiometric Ratio of A ReactionDerek Hammons100% (1)
- ACS Review: - Quick Refresher of Materials - Some Sample Questions and Short CutsDocument31 pagesACS Review: - Quick Refresher of Materials - Some Sample Questions and Short Cutsjhhjjh100% (1)
- IB Questionbank With ANSWERSDocument6 pagesIB Questionbank With ANSWERSRaunak ChawlaNo ratings yet
- Unit 2 Notes - Molecular & Ionic Compound Structure & PropertiesDocument18 pagesUnit 2 Notes - Molecular & Ionic Compound Structure & PropertiesDragonbariumNo ratings yet
- 5.3 (153 Marks) : MarkschemeDocument41 pages5.3 (153 Marks) : MarkschemeSemwezi EnockNo ratings yet
- Acids and BasesDocument45 pagesAcids and Basesjordanf48922100% (1)
- 2015 JC 2 H2 Hydroxyl Tutorial (Teachers)Document21 pages2015 JC 2 H2 Hydroxyl Tutorial (Teachers)JohnNo ratings yet
- Sch3u Test Review With AnswersDocument2 pagesSch3u Test Review With Answersapi-251470138No ratings yet
- IB Chemistry HL Test 2nd FEBDocument13 pagesIB Chemistry HL Test 2nd FEBprasad100% (1)
- Moles CalculationsDocument4 pagesMoles Calculationskjj7760No ratings yet
- 1.4 Energetics Revision QuestionsDocument88 pages1.4 Energetics Revision QuestionsTheMagicCarpet0% (1)
- Revision 12 IB Paper 1Document293 pagesRevision 12 IB Paper 1SiddharthChoksi100% (7)
- SCH4U - Unit 4 - Version CDocument45 pagesSCH4U - Unit 4 - Version CMr. SharpnNo ratings yet
- Organic Chemistry Study GuideDocument25 pagesOrganic Chemistry Study GuidemattNo ratings yet
- AP Chemistry - Oxidation Numbers PracticeDocument2 pagesAP Chemistry - Oxidation Numbers Practicemartialartsgrl21No ratings yet
- Limiting Reagent Worksheets #1-2Document4 pagesLimiting Reagent Worksheets #1-2gabrielaNo ratings yet
- IB Chemistry - SL - Chapter 10Document33 pagesIB Chemistry - SL - Chapter 10UltramixNo ratings yet
- IB Chemistry HL Summer AssignmentDocument4 pagesIB Chemistry HL Summer AssignmentVal B100% (1)
- Acid Base HomeworkDocument5 pagesAcid Base HomeworkAriel ChuNo ratings yet
- Stoichiometry UnitDocument56 pagesStoichiometry UnitCaiaphasNo ratings yet
- 8.1 (147 Marks) : MarkschemeDocument60 pages8.1 (147 Marks) : MarkschemeSemwezi EnockNo ratings yet
- Titration Curves: Strong Acid-Strong Base TitrationsDocument15 pagesTitration Curves: Strong Acid-Strong Base TitrationssandalailaNo ratings yet
- AP Chemistry: Chapter 7 - Atomic Structure & PeriodicityDocument14 pagesAP Chemistry: Chapter 7 - Atomic Structure & PeriodicityS. Green100% (1)
- 02 Petrucci10e CSMDocument33 pages02 Petrucci10e CSMAlexNo ratings yet
- Samora 3Document42 pagesSamora 3VovNo ratings yet
- Organic Chemistry Question IB Chem SLDocument40 pagesOrganic Chemistry Question IB Chem SLAarav Verma100% (1)
- 11.1 Multiple Choice and Bimodal Questions: Diff: 3 Page Ref: Sec. 11.2Document46 pages11.1 Multiple Choice and Bimodal Questions: Diff: 3 Page Ref: Sec. 11.2Katherine McLarney33% (3)
- 11U Chemistry Exam Review Questions (Part 1) Units 1 - 3Document9 pages11U Chemistry Exam Review Questions (Part 1) Units 1 - 3tareqrxNo ratings yet
- Topic 10 HLQDocument23 pagesTopic 10 HLQVũ Đức DuyNo ratings yet
- Ib Chemistry BondingDocument18 pagesIb Chemistry BondingAaron Bonner100% (1)
- Balancing EquationsDocument1 pageBalancing Equationslianchen251110No ratings yet
- Ib PPT 3 SL PDFDocument24 pagesIb PPT 3 SL PDFzarna nirmal rawalNo ratings yet
- Unit 4 Organic Chemistry ReactionsDocument6 pagesUnit 4 Organic Chemistry ReactionsRobbing_Hood100% (1)
- 15.1 (119 Marks) : MarkschemeDocument34 pages15.1 (119 Marks) : MarkschemeSemwezi Enock0% (1)
- HL Chemistry IA Checklist Updated Dec 2011Document6 pagesHL Chemistry IA Checklist Updated Dec 2011Karl Naumann100% (1)
- Sch3u Exam Review Ws s2018 PDFDocument4 pagesSch3u Exam Review Ws s2018 PDFwdsfNo ratings yet
- Energetics QuestionsDocument58 pagesEnergetics QuestionsQasim Peracha100% (1)
- Test Bank For Organic Chemistry 7th Edition by BrownDocument23 pagesTest Bank For Organic Chemistry 7th Edition by BrownandielanaNo ratings yet
- IB Chemistry IA Guidance and HintsDocument4 pagesIB Chemistry IA Guidance and HintsGinevraPiccioniNo ratings yet
- 7 Acid and BasesDocument27 pages7 Acid and BasessemalupurpleNo ratings yet
- Carbonyl Condensation ReactionsDocument41 pagesCarbonyl Condensation ReactionsVladislav PapperNo ratings yet
- 7.1 (149 Marks) : MarkschemeDocument51 pages7.1 (149 Marks) : MarkschemeSemwezi Enock100% (1)
- Chemistry 2 NotesDocument101 pagesChemistry 2 NotesAnna Conigrave100% (2)
- Acids Bases 1Document11 pagesAcids Bases 1Kelsey FarrugiaNo ratings yet
- 13.ionic Equilibria NotesDocument37 pages13.ionic Equilibria Notesgeoboom12100% (15)
- Chemistry SummaryDocument132 pagesChemistry SummarySebuta HuzaimaNo ratings yet
- Commonwealth Statutory Declaration Form (May 2011) PDFDocument2 pagesCommonwealth Statutory Declaration Form (May 2011) PDFcgao30No ratings yet
- Theme IV Yr 5d Content Guide - MatrixDocument9 pagesTheme IV Yr 5d Content Guide - Matrixcgao30No ratings yet
- CD Marker Panel Download 2Document2 pagesCD Marker Panel Download 2cgao30No ratings yet
- CPPREP4002 - Annotated Unit GuideDocument8 pagesCPPREP4002 - Annotated Unit Guidecgao30No ratings yet
- Student Elective Rotation GuideDocument3 pagesStudent Elective Rotation Guidecgao30No ratings yet
- Commonwealth Statutory Declaration Form (May 2011) PDFDocument2 pagesCommonwealth Statutory Declaration Form (May 2011) PDFcgao30No ratings yet
- Pythagoras WorksheetDocument2 pagesPythagoras Worksheetcgao30No ratings yet
- CD30 Draft CollationDocument8 pagesCD30 Draft Collationcgao30No ratings yet
- CD30 Literature Review PlanDocument1 pageCD30 Literature Review Plancgao30No ratings yet
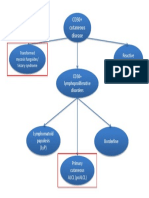
- CD30 Cutaneous Disease FlowchartDocument1 pageCD30 Cutaneous Disease Flowchartcgao30No ratings yet
- CD30 Literature Review PlanDocument1 pageCD30 Literature Review Plancgao30No ratings yet
- Lit Reviews For RX Students v7Document20 pagesLit Reviews For RX Students v7Joshua McdonaldNo ratings yet
- CD30 Literature Review PlanDocument1 pageCD30 Literature Review Plancgao30No ratings yet
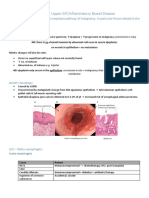
- Paediatrics: Acyanotic Heart DiseaseDocument5 pagesPaediatrics: Acyanotic Heart Diseasecgao30No ratings yet
- Matrix TopicsDocument3 pagesMatrix Topicscgao30No ratings yet
- Benign Lesions of The Vulva & Vagina (Table)Document3 pagesBenign Lesions of The Vulva & Vagina (Table)cgao30No ratings yet
- Pelvic Organ ProlapseDocument5 pagesPelvic Organ Prolapsecgao30No ratings yet
- Fibroids: 1. Red DegenerationDocument2 pagesFibroids: 1. Red Degenerationcgao30No ratings yet
- Pathology Lecture 7 - LiverDocument11 pagesPathology Lecture 7 - Livercgao30No ratings yet
- Preterm LabourDocument3 pagesPreterm Labourcgao30No ratings yet
- EndometriosisDocument1 pageEndometriosiscgao30No ratings yet
- Skin Terms: DescriptorsDocument2 pagesSkin Terms: Descriptorscgao30No ratings yet
- O&G GlossaryDocument3 pagesO&G Glossarycgao30No ratings yet
- Pathology Lecture 5 - Upper GITDocument10 pagesPathology Lecture 5 - Upper GITcgao30No ratings yet
- Intro To Motor Systems: (Why Else Would You Go To The Gym, Right?)Document13 pagesIntro To Motor Systems: (Why Else Would You Go To The Gym, Right?)cgao30No ratings yet
- Dna Notes (3&7) - CompleteDocument11 pagesDna Notes (3&7) - Completecgao30100% (1)
- Pathology Lecture 2 - NeoplasiaDocument15 pagesPathology Lecture 2 - Neoplasiacgao30No ratings yet
- Pathology Lecture 7 - LiverDocument11 pagesPathology Lecture 7 - Livercgao30No ratings yet
- Inflammatory Conditions (Disease Mechanisms)Document3 pagesInflammatory Conditions (Disease Mechanisms)cgao30No ratings yet
- Cell Respiration Notes (3&8)Document9 pagesCell Respiration Notes (3&8)cgao30No ratings yet
- Extracting MetalsDocument14 pagesExtracting MetalsSusan ChongNo ratings yet
- List of Codex Specifications For Food Additives (CAC/MISC 6-2012)Document107 pagesList of Codex Specifications For Food Additives (CAC/MISC 6-2012)Larisa Mihaela GiuraNo ratings yet
- Volcanogenic Massive Sulfide Deposits: MD Hannington, University of Ottawa, Ottawa, ON, CanadaDocument26 pagesVolcanogenic Massive Sulfide Deposits: MD Hannington, University of Ottawa, Ottawa, ON, CanadaAbraham Alejandro Arana VilcaNo ratings yet
- Types of AlloyDocument2 pagesTypes of AlloyOliver Reidsil M. RojalesNo ratings yet
- MME Group Anode - BookletDocument137 pagesMME Group Anode - BookletvtechelectricNo ratings yet
- Alternate Building MaterialsDocument36 pagesAlternate Building MaterialsramNo ratings yet
- Cen Iso TR 20172 EnglishDocument31 pagesCen Iso TR 20172 EnglishMihai Nistor100% (1)
- Milling Cutter: Prepared By:-Prof. Varpe N. JDocument24 pagesMilling Cutter: Prepared By:-Prof. Varpe N. Jsample useNo ratings yet
- 9th Atoms and Molecules Chemistry Test PaperDocument3 pages9th Atoms and Molecules Chemistry Test Paperanupamkhanna100% (1)
- Organic Chemistry PracticalDocument9 pagesOrganic Chemistry PracticalAbdulmujeeb simpaNo ratings yet
- Innermost - Main CatalogueDocument214 pagesInnermost - Main CatalogueLiteHouseNo ratings yet
- P1 - The Periodic TableDocument20 pagesP1 - The Periodic TableEdgardo LeysaNo ratings yet
- ABB CorrosionDocument8 pagesABB CorrosionForexFFNo ratings yet
- Copper LeachingDocument13 pagesCopper LeachingLutfi ムハンマドNo ratings yet
- Friction WeldingDocument49 pagesFriction WeldingSACHIN A. MORE100% (1)
- Mechanical QuizDocument56 pagesMechanical QuizsigmasundarNo ratings yet
- Chapter Assessment The Periodic Table and Periodic Law Student EditableDocument8 pagesChapter Assessment The Periodic Table and Periodic Law Student Editableanon_789010972No ratings yet
- BASF Mining Solutions Product RangeDocument2 pagesBASF Mining Solutions Product RangePrototypeNo ratings yet
- Dental Materials (Review Center)Document11 pagesDental Materials (Review Center)yellow rangerNo ratings yet
- Calibration Vulcan ExpertDocument6 pagesCalibration Vulcan Expertsoufiane el khomssiNo ratings yet
- Buffoli Booklet - USA PhoscoatingDocument138 pagesBuffoli Booklet - USA PhoscoatingMark GarrettNo ratings yet
- Biochem Lab ManualDocument5 pagesBiochem Lab ManualshaneskiranrajaNo ratings yet
- SS-CRM No. 493/3 High Manganese Steel: Certificate of AnalysisDocument2 pagesSS-CRM No. 493/3 High Manganese Steel: Certificate of Analysislehdruk7100No ratings yet
- Arcam ASTM F75 Cobalt Chrome PDFDocument3 pagesArcam ASTM F75 Cobalt Chrome PDFboni_briantoniNo ratings yet
- CeramicDocument6 pagesCeramicHananNo ratings yet
- 3196 I FRAME BulletinDocument19 pages3196 I FRAME BulletinLuis CuaxiloNo ratings yet
- Head Office: 1/3-H-A-2, Street # 6, East Azad Nagar, Delhi-110051 (One KM From Welcome Metro Station)Document6 pagesHead Office: 1/3-H-A-2, Street # 6, East Azad Nagar, Delhi-110051 (One KM From Welcome Metro Station)Vikash SharmaNo ratings yet
- 0620 w07 QP 3Document16 pages0620 w07 QP 3Haider AliNo ratings yet
- Equinox International LTD - Stainless Steel - ST ST Corrosion Resistance - 106 PDFDocument2 pagesEquinox International LTD - Stainless Steel - ST ST Corrosion Resistance - 106 PDFeugenio.gutenbertNo ratings yet
- Blast FurnaceDocument91 pagesBlast FurnaceSarbajitManna100% (1)