Professional Documents
Culture Documents
Solving Enzyme Kinetics Problems: Sucrose Transport, Vmax vs Km Effects, Carbonic Anhydrase Reaction
Uploaded by
biotech_vidhya100%(1)100% found this document useful (1 vote)
65 views4 pagesscience
Original Title
3
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentscience
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
65 views4 pagesSolving Enzyme Kinetics Problems: Sucrose Transport, Vmax vs Km Effects, Carbonic Anhydrase Reaction
Uploaded by
biotech_vidhyascience
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 4
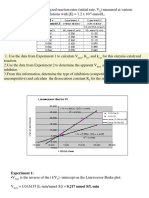
5/28/2014 3.
5 Solving Enzyme Kinetics Problems
http://202.114.65.51/fzjx/wsw/website/mit/eb/kinetics/solvingkinetics.html 1/4
3.5 Solving Enzyme Kinetics Problems
1) Two strains of Bacterium sweetans, A and B, use sucrose (table sugar) as a sole carbon source. The first step
in the process of sucrose utilization is the passage of sucrose through a sucrose transporter protein in the
membrane. The characteristics of the two transport proteins are as follows (assuming [E]tot is the same in both):
Strain A B
KM 1000 mM 10 mM
Vmax 1000 mmol/min 100 mmol/min
a) Assuming that the rate of sucrose uptake is the rate limiting step in growth, which strain will grow faster
if the concentration of glucose is: 10 mM? 100 mM? 1000 mM?
b) One strain was isolated from the soil and the other from the floor at Toscanini's Ice Cream, which was
likely to be which? Why?
Solution:
Using the Michaelis-Menten equation, you can calculate the initial velocity of sucrose uptake for each strain
under the conditions listed. Note that this is the initial rate, as sucrose was depleted from the medium, the rate
would decrease. To simplify matters, assume that the number of cells is small so that the sucrose concentration
does not change appreciably during growth, so that the transport protein is always running at Vo.
For these calculations, substrate = sucrose.
Vo, enzyme of A: Vo, enzyme of B: Faster
[sucrose] Km=1000mM Km= 10mM Growing
mM Vmax=1000mmol/min Vmax= 100mmol/min strain:
-------------------------------------------------------------------------------
10 9.9 mmol/min 50 mmol/min B
100 91 mmol/min 91 mmol/min neither
1000 500 mmol/min 99 mmol/min A
b) It is likely that the [sucrose] levels in the soil are very low, so that strain B would be at a selective advantage
there. It is likely that a soil bacterium would have transport machinery adapted for low [sucrose].
It is likely that the [sucrose] on the floor at Toscanini's has a much higher [sucrose] than the soil. Under these
conditions, strain A would be at an advantage.
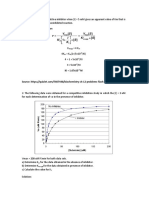
2) Given an enzyme with a Km = 10 mM and Vmax = 100 mmol/min.
a) If [S]=100mM, which will increase the velocity more: a 10-fold decrease in Km, or a 10-fold increase
in Vmax?
b) If [S]=10mM, which will increase the velocity more: a 10-fold decrease in Km, or a 10-fold increase in
Vmax?
5/28/2014 3.5 Solving Enzyme Kinetics Problems
http://202.114.65.51/fzjx/wsw/website/mit/eb/kinetics/solvingkinetics.html 2/4
Solution:
You can answer these questions qualitatively or quantitatively. Having a qualitative understanding of what KM
and Vmax mean is more important than memorizing the Michaelis-Menten equation.
Qualitatively:
a) With [S]>>KM, the enzyme is running close to Vmax . Because of this, decreasing KM would hardly
increase V at all, but increasing Vmax 10-fold would increase V 10-fold.
b) With [S] = Km, the enzyme is running at 50% of Vmax. Decreasing Km 10-fold will raise V to near
Vmax, doubling V at most. Increasing Vmax 10-fold would also increase V 10-fold.
Quantitatively:
part Km Vmax [S] Vo result
-----------------------------------------------------------------------
a 10 mM 100 mmol/min 100 mM 91 mmol/min
a 1 mM 100 mmol/min 100 mM 99 mmol/min
a 10 mM 1000 mmol/min 100 mM 910 mmol/min greater effect
b 10 mM 100 mmol/min 10 mM 50 mmol/min
b 1 mM 100 mmol/min 10 mM 91 mmol/min
b 10 mM 1000 mmol/min 10 mM 500 mmol/min greater effect
3)The enzyme Carbonic Anhydrase (CA) catalyzes the following reaction:
CO2 + H20 <-----------> H2CO3
Given two identical sealed tubes with CO2 gas above buffer containing H2O and CO2 - one with added
carbonic anhydrase, one without. Allow the contents to come to equilibrium, then:
a) At equilibrium, which tube will have more CO2 in the gas phase, A or B?
b) Add CO2 to the gas phase of both tubes. Which tube will absorb it faster into the liquid, A or B?
Why?
c) Remove CO2 from the gas phase of both tubes. Which will replenish the CO2 faster, A or B? Why?
d) Take tube A, allow it to come to equilibrium, and inject a negligible volume of highly concentrated CA
into the liquid layer. Will CO2 gas be produced, absorbed, or will there be no change?
5/28/2014 3.5 Solving Enzyme Kinetics Problems
http://202.114.65.51/fzjx/wsw/website/mit/eb/kinetics/solvingkinetics.html 3/4
e) Take tube B, allow it to come to equilibrium, and inject a negligible volume of highly concentrated ATP.
Will CO2 gas be produced, absorbed, or will there be no change?
Solution:
a) Since the tubes are at equilibrium, and since enzymes do not change the equilibrium concentrations of
reactants and products (another way of saying that they do not change Delta G), both tubes will have
identical [CO2] in the gas phase.
b) The CA in tube B will accelerate the absorption of CO2 by accelerating the conversion of CO2 into
H2CO3.
c) The CA in tube B will accelerate the production of CO2 by accelerating the conversion of H2CO3 into
CO2.
d) This is like part A, the CA cannot affect the equilibrium concentrations of reactants or products, only
alter the rate. So addition of CA to a tube at equilibrium will have no effect.
{However, addition of CA to a tube that was not at equilibrium could cause rapid absorption or
production of CO2. Normally, enzyme assays are carried out in reactions where the substrates have not
had enough time to reach equilibrium, so that the effect of adding enzyme will be detectable.}
e) Nothing will happen. Since ATP is not a substrate of CA, the concentrations of reactants and products
have not changed, so the equilibrium will not shift.
{If ATP were a substrate of CA, then adding it to the reaction would change the amount of CO2 in the
gas phase.}
4) The enzyme Lysozyme is present in human nasal mucus and catalyzes the breakdown of a component of the
cell wall of certain bacteria, causing them to burst. Lysozyme cleaves a polymer of N-acetyl-glucosamine
(NAG)n into smaller fragments:
lysozyme
NAG-NAG-NAG-NAG-NAG-NAG-NAG-NAG-NAG-NAG-NAG-NAG + H2O --------->
NAG-NAG-NAG-NAG-NAG-NAG + NAG-NAG-NAG-NAG-NAG-NAG
a) Given that the reaction proceeds as written, what can you conclude about the relative stability of
(NAG)n?
b) Given the following information, what can you conclude about the binding of lysozyme to its substrate?
Substrate Relative rate of hydrolysis
(NAG)2 0
(NAG)3 1
(NAG)4 8
(NAG)5 4,000
(NAG)6 30,000
5/28/2014 3.5 Solving Enzyme Kinetics Problems
http://202.114.65.51/fzjx/wsw/website/mit/eb/kinetics/solvingkinetics.html 4/4
(NAG)8 30,000
c) If you treated a solution of (NAG)100 with lysozyme, what would the majority of the product be?
Solution:
a) Since lysozyme cannot effect the Delta G of the reaction, (NAG)n must be unstable (that is, the
reaction where it breaks into monomers has a Delta G<0).
{But why then do bacteria use an unstable molecule as a cell wall? Because, even though the (NAG)n will
eventually come to equilibrium and decompose into monomers, the uncatalyzed rate of hydrolysis is very
low, so reaching equilibrium might take decades. Lysozyme increases this rate enormously, and acts as a
bacteriocide.}
b) Even though the reaction catalyzed by lysozyme appears to be:
NAG-NAG + H2O --------> NAG + NAG
the substrate binding site must have a pocket designed to hold 5 or 6 NAG units before the enzyme can
get into the active conformation. This is an example of substrate specificity.
c) After a short time, it would mostly be (NAG)4, since any larger fragments will be rapidly cleaved. After
a long time, it will be (NAG)2 and (NAG)3.
shanec@mit.edu
You might also like
- Enzyme KineticsDocument3 pagesEnzyme KineticsZeny Naranjo100% (2)
- Solutions for Selected End of Chapter 6 ProblemsDocument6 pagesSolutions for Selected End of Chapter 6 Problemsheyyyale100% (1)
- Kinetic Vs Chemical MechanismDocument34 pagesKinetic Vs Chemical MechanismIgnacio Bascuñán OyarceNo ratings yet
- Assign#2 Enzyme KineticsDocument3 pagesAssign#2 Enzyme KineticsKaren Castro100% (2)
- 2-Bacterial Growth Kinetics - F11Document15 pages2-Bacterial Growth Kinetics - F11Suvidha Chib100% (1)
- Cell Growth Kinetics BioprosesDocument24 pagesCell Growth Kinetics BioprosesLuthfi Magecho Sikoembang100% (1)
- Lecture Notes-Growth Kinetics - Growth PhasesDocument24 pagesLecture Notes-Growth Kinetics - Growth PhasesNhan Nguyen100% (1)
- MIT Lecture 6 5.07 Biochemistry LectureDocument16 pagesMIT Lecture 6 5.07 Biochemistry LectureakiridoNo ratings yet
- MORE Practice For Michaelis-Menten ShitDocument3 pagesMORE Practice For Michaelis-Menten ShitMalcolm Charles100% (1)
- Monod Equation ProblemDocument7 pagesMonod Equation Problemeiddnew100% (1)
- Enzyme KineticsDocument72 pagesEnzyme Kineticsitokki otoya100% (1)
- Growth Kinetics CalculationDocument24 pagesGrowth Kinetics CalculationSarah Pavu100% (1)
- Problem Set On Enzyme Kinetics - FS - 2012 - 2013Document2 pagesProblem Set On Enzyme Kinetics - FS - 2012 - 2013Chay Alcantara100% (1)
- BIO307 Lecture 5 (Enzyme Kinetics I)Document11 pagesBIO307 Lecture 5 (Enzyme Kinetics I)Phenyo Mmereki100% (1)
- 7-Bioc431 Enzymes Kinetics S15Document34 pages7-Bioc431 Enzymes Kinetics S15Shariq Mansoor KhanNo ratings yet
- Sample Exam 4 Fall 11Document14 pagesSample Exam 4 Fall 11janohxNo ratings yet
- Che503 PS3 PDFDocument2 pagesChe503 PS3 PDFCarissa TejioNo ratings yet
- Problem Set - Enzymes From LehningerDocument11 pagesProblem Set - Enzymes From LehningervioletbrownNo ratings yet
- Excel 2007 TutorialDocument5 pagesExcel 2007 TutorialromelcarvajalNo ratings yet
- 5 Enzyme Kinetics-InhibitionDocument40 pages5 Enzyme Kinetics-InhibitionJoel SmolanoffNo ratings yet
- Microbial Cell Growth and Kinetics PhasesDocument58 pagesMicrobial Cell Growth and Kinetics PhasesKIL170051 STUDENTNo ratings yet
- Tutorial 1Document2 pagesTutorial 1eddy50% (2)
- Question CH06+answer PDFDocument8 pagesQuestion CH06+answer PDFCris-Anne Juangco III100% (1)
- Lecture Notes Enzyme 2 Enzyme Kinetics WebDocument29 pagesLecture Notes Enzyme 2 Enzyme Kinetics WebAldren RebaLdeNo ratings yet
- Statistics Study Guide Chi-SquareDocument4 pagesStatistics Study Guide Chi-SquareldlewisNo ratings yet
- Cell Biology Chapter Links & Exam FlashcardsDocument3 pagesCell Biology Chapter Links & Exam FlashcardsSam Khader100% (1)
- Optimization of Cellulase Enzyme From Vegetable Waste by Using Trichoderma Atroviride in Solid State FermentationDocument6 pagesOptimization of Cellulase Enzyme From Vegetable Waste by Using Trichoderma Atroviride in Solid State FermentationIOSRjournalNo ratings yet
- Stefan Boltzmann Law PDFDocument3 pagesStefan Boltzmann Law PDFESAKKIMALA SNo ratings yet
- Biochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For ExaminationDocument11 pagesBiochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For ExaminationJenna ReynoldsNo ratings yet
- Bioreactors: BY Agomuoh Paul Kelechi 20111200 Cyprus International University DEC 27, 2011Document23 pagesBioreactors: BY Agomuoh Paul Kelechi 20111200 Cyprus International University DEC 27, 2011Rommel AguillonNo ratings yet
- ENZYME KINETICSDocument11 pagesENZYME KINETICSDianne Villanueva100% (1)
- Enzym ESDocument21 pagesEnzym ESpoopnoodlemanNo ratings yet
- Enzyme InhibitionDocument7 pagesEnzyme InhibitionJane Docdoc100% (1)
- STRAIN IMPROVEMENT TechniquesDocument28 pagesSTRAIN IMPROVEMENT TechniqueselaiyarajaNo ratings yet
- Growth Rate and Yield Calculations - 17.11.16Document10 pagesGrowth Rate and Yield Calculations - 17.11.16Raghav Suresh100% (1)
- Sutherland 1991Document7 pagesSutherland 1991Isal AbdussalamNo ratings yet
- Biosepartaion Engineering: Ch.1: Bioseparation & Biological MaterialsDocument89 pagesBiosepartaion Engineering: Ch.1: Bioseparation & Biological MaterialsAlex MaximusNo ratings yet
- Lecture Notes-Growth Kinetics - Growth PhasesDocument24 pagesLecture Notes-Growth Kinetics - Growth Phasesbioenviron100% (1)
- Chem 40 Enzyme KineticsDocument85 pagesChem 40 Enzyme KineticsJustine Grace Mariano100% (1)
- Classification of BioreactorsDocument2 pagesClassification of BioreactorsisabelelmhNo ratings yet
- Name: Kristine Joy Atos Block: BSN 1-D Practice ProblemsDocument6 pagesName: Kristine Joy Atos Block: BSN 1-D Practice ProblemsJenz Hope Segui Novela100% (3)
- Enzyme velocity at 1/4 Vmax with 0.5mM KMDocument1 pageEnzyme velocity at 1/4 Vmax with 0.5mM KMfintastellaNo ratings yet
- Stoichiometry of Microbial Growth and Product FormationDocument30 pagesStoichiometry of Microbial Growth and Product FormationMark GerardNo ratings yet
- (Springer Laboratory) Prof. Sadao Mori, Dr. Howard G. Barth (Auth.) - Size Exclusion Chromatography-Springer-Verlag Berlin Heidelberg (1999)Document235 pages(Springer Laboratory) Prof. Sadao Mori, Dr. Howard G. Barth (Auth.) - Size Exclusion Chromatography-Springer-Verlag Berlin Heidelberg (1999)Luis Paulo BernardiNo ratings yet
- Chapter 10 Chemical Kinetics IIDocument131 pagesChapter 10 Chemical Kinetics IIChicken ChickenNo ratings yet
- Amylase Assay 2Document9 pagesAmylase Assay 2Rahman ImudaNo ratings yet
- First Year Medics TestDocument15 pagesFirst Year Medics TestJosephNo ratings yet
- Enzyme Kinetics Examples and ProblemsDocument4 pagesEnzyme Kinetics Examples and Problemskiiadizon07100% (1)
- A.1. Competitive InhibitionDocument5 pagesA.1. Competitive InhibitionFlorecita Cabañog100% (1)
- Voet - Chapt - 12 Properties of EnzymesDocument102 pagesVoet - Chapt - 12 Properties of Enzymestelmo flowNo ratings yet
- Enzyme Immobilization Methods and EffectsDocument3 pagesEnzyme Immobilization Methods and Effectsfintastella0% (1)
- 1.carbohydrates and Lipid Metabolism-Converted - WatermarkDocument97 pages1.carbohydrates and Lipid Metabolism-Converted - WatermarkJuliyamol JoseNo ratings yet
- HOW TO - Calculate KM and Vmax With ExcelDocument3 pagesHOW TO - Calculate KM and Vmax With Excelminjeshp67% (3)
- Enzyme Kinetics Questions and Answers: 2 Year Undergraduates-Biology 2018-2019Document8 pagesEnzyme Kinetics Questions and Answers: 2 Year Undergraduates-Biology 2018-2019Emmanuel JoyNo ratings yet
- The Structure and Function of MacromoleculesDocument70 pagesThe Structure and Function of MacromoleculesLustried Nadyang100% (1)
- Chapter 2 Protein SeparationDocument83 pagesChapter 2 Protein SeparationZul Adli SaribinNo ratings yet
- Molecular Pharming: Applications, Challenges and Emerging AreasFrom EverandMolecular Pharming: Applications, Challenges and Emerging AreasAllison R. KermodeNo ratings yet
- Membrane Research: Classic Origins and Current ConceptsFrom EverandMembrane Research: Classic Origins and Current ConceptsA. L. Muggleton-HarrisNo ratings yet
- Facs ProtocolDocument7 pagesFacs ProtocolmisterxNo ratings yet
- BT 2019Document13 pagesBT 2019biotech_vidhyaNo ratings yet
- Q.No. Type Section Key/Range MarksDocument3 pagesQ.No. Type Section Key/Range Marksbiotech_vidhyaNo ratings yet
- Components Reaction MixtureDocument3 pagesComponents Reaction Mixturebiotech_vidhyaNo ratings yet
- Stripping For ReprobingDocument2 pagesStripping For ReprobingStella SalvatoreNo ratings yet
- Troubleshooting SDS-PAGE 1Document3 pagesTroubleshooting SDS-PAGE 1biotech_vidhyaNo ratings yet
- Stripping For ReprobingDocument2 pagesStripping For ReprobingStella SalvatoreNo ratings yet
- SDS PageDocument2 pagesSDS Pagebiotech_vidhyaNo ratings yet
- Nuclear ExtractsDocument2 pagesNuclear Extractsbiotech_vidhyaNo ratings yet
- Buffer Preparation Guide for DNA/Protein Work (Shi LabDocument6 pagesBuffer Preparation Guide for DNA/Protein Work (Shi Labbiotech_vidhyaNo ratings yet
- Polymerasen GuideDocument16 pagesPolymerasen Guidebiotech_vidhyaNo ratings yet
- Buffer Preparation Guide for DNA/Protein Work (Shi LabDocument6 pagesBuffer Preparation Guide for DNA/Protein Work (Shi Labbiotech_vidhyaNo ratings yet
- Polymerase Chain Reaction (PCR)Document3 pagesPolymerase Chain Reaction (PCR)biotech_vidhyaNo ratings yet
- Brad FordDocument12 pagesBrad FordQi ChaoNo ratings yet
- Whole Cell ExtractDocument1 pageWhole Cell Extractbiotech_vidhyaNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- A.E. (Mechanical Engineering I) 2007Document24 pagesA.E. (Mechanical Engineering I) 2007Mukesh KumarNo ratings yet
- Qpaper PondyDocument21 pagesQpaper Pondybiotech_vidhyaNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- Ies 17 Set A Me Q ADocument67 pagesIes 17 Set A Me Q Abiotech_vidhyaNo ratings yet
- Part and Mold Design GuideDocument170 pagesPart and Mold Design GuideminhtintinNo ratings yet
- Qpaper PondyDocument21 pagesQpaper Pondybiotech_vidhyaNo ratings yet
- Img Word-To PDFDocument3 pagesImg Word-To PDFbiotech_vidhyaNo ratings yet
- TNPSC Group 1 Prelim Book List PDFDocument2 pagesTNPSC Group 1 Prelim Book List PDFbiotech_vidhyaNo ratings yet
- ESE 2017 Mechanical Engineering Prelims Exam Detailed SolutionDocument52 pagesESE 2017 Mechanical Engineering Prelims Exam Detailed SolutionpataNo ratings yet
- Mechanical Engineering Code No. 14: Combined Competitive (Preliminary) Examination, 2010Document20 pagesMechanical Engineering Code No. 14: Combined Competitive (Preliminary) Examination, 2010biotech_vidhyaNo ratings yet
- Recruitment RulesDocument5 pagesRecruitment Rulesbiotech_vidhyaNo ratings yet
- 1 TolerancesDocument1 page1 Tolerancesbiotech_vidhyaNo ratings yet
- TDC 41597 A (Mechanical Engg.) - 2012Document20 pagesTDC 41597 A (Mechanical Engg.) - 2012biotech_vidhyaNo ratings yet
- Befcv List PDFDocument22 pagesBefcv List PDFbiotech_vidhyaNo ratings yet
- Lab 1Document7 pagesLab 1attiqueNo ratings yet
- Brchembase LRDocument4 pagesBrchembase LRRavindra PawarNo ratings yet
- Biological MoleculesDocument98 pagesBiological MoleculesSuyashNo ratings yet
- Daily Lesson Log TemplateDocument64 pagesDaily Lesson Log TemplateArlene ChavezNo ratings yet
- JIS G3466 - Thailand - CONTENT1011579693989745Document1 pageJIS G3466 - Thailand - CONTENT1011579693989745Nguyễn Tiến TùngNo ratings yet
- Relativity: The Special and General TheoryDocument11 pagesRelativity: The Special and General TheoryKhamsah Al-FarhanNo ratings yet
- Asam Mefenamat EmulgelDocument5 pagesAsam Mefenamat EmulgelVi Vian HiuNo ratings yet
- 2.0 Electric FieldsDocument4 pages2.0 Electric FieldsEdAnNo ratings yet
- Ionic EquilibriumDocument91 pagesIonic EquilibriumGabrielNo ratings yet
- Welding ProcessesDocument46 pagesWelding Processesbabitasharma100% (1)
- What Is Organic ChemistryDocument4 pagesWhat Is Organic Chemistrybas haNo ratings yet
- 40nicrmo7-3: Steel GradeDocument3 pages40nicrmo7-3: Steel GradeGanesh K CNo ratings yet
- BurnerDocument33 pagesBurnertoficNo ratings yet
- Gas Circulation - Cement Plant PDFDocument127 pagesGas Circulation - Cement Plant PDFKenny RuizNo ratings yet
- Determining The Chemical Resistance of Concrete Products To Acid AttackDocument4 pagesDetermining The Chemical Resistance of Concrete Products To Acid AttackYoshi TaissonNo ratings yet
- What Is Pyrophoric Iron OxidationDocument9 pagesWhat Is Pyrophoric Iron OxidationGhuna Uciha100% (1)
- Crude Oil Assay Database - Crude Oil Data Properties and Definitions - KnovelDocument6 pagesCrude Oil Assay Database - Crude Oil Data Properties and Definitions - KnovelValeanu ErmilNo ratings yet
- Manual de PLT NeilDocument172 pagesManual de PLT Neilfergot2010No ratings yet
- Nanoparticle LabDocument12 pagesNanoparticle Labglen-576661No ratings yet
- Reactor Design Sample ExamDocument7 pagesReactor Design Sample ExamAugustine BrockNo ratings yet
- Zumdahl Chapter 9Document24 pagesZumdahl Chapter 9Master NistroNo ratings yet
- TH-L32C10R2: Model NoDocument121 pagesTH-L32C10R2: Model Nozerson13No ratings yet
- Alambre SoudokayDocument8 pagesAlambre SoudokayEzequielNo ratings yet
- Module-3.2 Sieve Tray Design 8Document56 pagesModule-3.2 Sieve Tray Design 8Harsh Garg 24601No ratings yet
- Migration From MAterials in Contact With Food StuffsDocument32 pagesMigration From MAterials in Contact With Food Stuffsmohd shahrukhNo ratings yet
- Direct Numerical Simulations of Non-Equilibrium Dynamics of ColloidsDocument33 pagesDirect Numerical Simulations of Non-Equilibrium Dynamics of ColloidsmortezagashtiNo ratings yet
- Hazardous Materials Table 172 - 101tbDocument211 pagesHazardous Materials Table 172 - 101tbZeero AndoneNo ratings yet
- 01 StudyGuide (2021) ChemDocument157 pages01 StudyGuide (2021) ChemYzakRVNo ratings yet
- Camphor MAR CAS No 76-22-2: Material Safety Data Sheet Sds/MsdsDocument7 pagesCamphor MAR CAS No 76-22-2: Material Safety Data Sheet Sds/MsdsArlan ZulkarnainNo ratings yet
- Thermo Fisher Scientific: Validation ReportDocument20 pagesThermo Fisher Scientific: Validation ReportAlexandra FloreaNo ratings yet