Professional Documents
Culture Documents
N, N-Diethyl M-Toluamide (OFF)
Uploaded by
Denisse Watt Cuarteros0 ratings0% found this document useful (0 votes)
220 views3 pagesThis lab report contains the detailed description on how the OFF (deet) was obtained and how it was analyzed. It also contains other information related to N, N-diethyl m-toluamide and how it was formed.
Original Title
N, N-diethyl m-toluamide (OFF)
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis lab report contains the detailed description on how the OFF (deet) was obtained and how it was analyzed. It also contains other information related to N, N-diethyl m-toluamide and how it was formed.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
220 views3 pagesN, N-Diethyl M-Toluamide (OFF)
Uploaded by
Denisse Watt CuarterosThis lab report contains the detailed description on how the OFF (deet) was obtained and how it was analyzed. It also contains other information related to N, N-diethyl m-toluamide and how it was formed.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
1
OFF, an Insect Repellant
The first step in order to synthesize OFF is by performing the reaction of the m-toluic
acid with the thionyl chloride. M-toluic acid has a high melting point that is why it is solid in
room temperature while thionyl chloride is a liquid which is usually used to convert the
carboxylic acids into their acid chloride derivatives. The reason why thionyl chloride can convert
acids into their acid chloride derivatives is because it is highly reactive. The S=O bond is highly
polarized and is analogous to the carbonyl bond. Also, the partial positive charge on the S is
susceptible to a nucleophilic attack. After the formation of an intermediate and removal of
proton, the result of the first-step of the reaction is the conversion of the poor leaving group (-
OH) into a good leaving group. The next reaction is to form the N, N-dimethyl toluamide by
reacting it with the acid chloride formed from the first reaction, with diethylamine. Diethylamine
is a good nucleophile and the carbonyl group is nicely polarized but the reason why it is not
advisable in this reaction to perform a reaction of carboxylic acid and diethylamine is because
the result of acid-base chemistry is a salt. In this second step of the reaction, the chloride anion
acts as the nucleophile. The chloride attack leads to a mini chain reaction which results in the
elimination of sulfur dioxide and hydrogen chloride. The acid chloride formed is much more
reactive than the carboxylic acid because chloride is a better leaving group. In reaction with the
acid chloride, the diethylamine acts as the nucleophile and attack at the partial positive charge of
the carbon of the acid chloride. A tetrahedral intermediate will then form. Then, the formation of
the C=O bond pushes out the best leaving group which is the chloride to form the N, N-dimethyl
toluamide.
During the work-up procedure, several extractions were made to get the desired product.
The first and second extraction procedure involves the addition of 5% aqueous NaOH to the
mixture to allow the layers to separate into two layers; top layer which contains the ether solution
2
and the desired product (organic layer), and the bottom layer which contains the aqueous layer.
The aqueous layer which is composed of unreacted m-toluic acid, traces of HCl, unreacted m-
toluic acid chloride, diethylamine, and NaCl are separated from the top layer and most of it were
removed from the organic layer of the solution. Then, 2 mL of 10% HCl aqueous solution was
then used to react with any remaining diethylamine to form hydrochloride salt which is also then
removed, by extracting the aqueous layer and leaving the organic layer. 2 mL of water was then
added to extract the remaining diethylamine hydrochloride, and remaining thionyl chloride.
Sodium sulfate, a drying agent which is used in acidic conditions, was then added so that the
ether solution that contains the desired product, N, N-diethyl toluamide, will turn clear and the
desired product will be more pure.
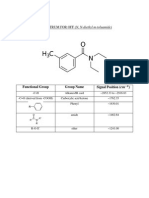
According to the IR spectra of the product obtained from the experiment, its IR spectrum
successfully identified the important peaks such as: phenyl group at ~1630.01
, amide peak
at ~1462.64
, carboxylic acid ketone at ~1762.35
, ether peak at ~1241.00
, and
the IR card/alkanes at ~2852.33
to ~2916.83
. The IR spectrum of the product
doesnt contain any OH peak which means that the product obtained is a pure N, N-diethyl
toluamide because it doesnt contain any alcohol nor water contamination. With the IR spectra
obtained and the important peaks identified, the synthesized product is no doubt going to work as
a mosquito repellant, OFF.
3
References
Bruice, P. Organic Chemistry, 7
th
ed.; Pearson: New Jersey, 2013.
Padias, A. Making The Connections, 2nd ed.; Hayden-McNeil Publishing: Plymouth, MI, 2013.
Padias, A. Organic Chemistry Laboratory Manual, 4th ed.; Hayden-McNeil Publishing:
Plymouth, MI, 2013.
You might also like
- LabDocument7 pagesLabLiz HackettNo ratings yet
- Organic Chemistry II Experiment Sodium Borohydride ReductionDocument10 pagesOrganic Chemistry II Experiment Sodium Borohydride ReductionAlohaaSwezzNo ratings yet
- Synthesis of Tert-Butyl Chloride from AlcoholDocument7 pagesSynthesis of Tert-Butyl Chloride from AlcoholFerdinand Tamayo Cayabyab Jr.No ratings yet
- Carbonyl CompoundsDocument40 pagesCarbonyl CompoundsMiguelNo ratings yet
- Synthesize Alkyl HalideDocument6 pagesSynthesize Alkyl HalideAnna Sophia EbuenNo ratings yet
- SYNTHESISDocument7 pagesSYNTHESISJhonis Bentes MeirellesNo ratings yet
- Adipic Acid PreparationDocument9 pagesAdipic Acid PreparationSmaeUB100% (1)
- Alkyl Thiolsulfinates Synthesized and CharacterizedDocument4 pagesAlkyl Thiolsulfinates Synthesized and CharacterizedmakajonaNo ratings yet
- Sodium Boronhydride Reduction of CyclohexanoneDocument6 pagesSodium Boronhydride Reduction of CyclohexanoneWan Nur Amira91% (11)
- Exp 3-Reduction of Cyclohexanone With Sodium BorohydrideDocument11 pagesExp 3-Reduction of Cyclohexanone With Sodium Borohydrideakuserai100% (3)
- A Green Method For Synthesis of Cyclohexanone Oxidation of Cyclohexanol Using Sodium HypochloriteDocument6 pagesA Green Method For Synthesis of Cyclohexanone Oxidation of Cyclohexanol Using Sodium Hypochloritefleetfoxes886% (7)
- Formal Report For Synthesis of An Alkyl HalideDocument5 pagesFormal Report For Synthesis of An Alkyl HalideLovelyn Marie Morada Nievales80% (5)
- Carbonyl Compounds: A2 Chemistry Unit 4Document45 pagesCarbonyl Compounds: A2 Chemistry Unit 4Faddy Oraha100% (1)
- Unit 4 Organic Chemistry ReactionsDocument6 pagesUnit 4 Organic Chemistry ReactionsRobbing_Hood100% (1)
- Mild Adipic Acid SynthesisDocument5 pagesMild Adipic Acid Synthesiskhaledegy10No ratings yet
- CHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneDocument5 pagesCHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneAmirul Azhar100% (1)
- 4.1.2 Carbonyl CompoundsDocument5 pages4.1.2 Carbonyl CompoundsFin BrickmanNo ratings yet
- Notes PharmaDocument24 pagesNotes Pharmashubhyog PawarNo ratings yet
- Cyclohexene Synthesis via Cyclohexanol DehydrationDocument3 pagesCyclohexene Synthesis via Cyclohexanol DehydrationImani London Smith67% (3)
- CHEM35.1 E7 Cannizzaro ReactionDocument4 pagesCHEM35.1 E7 Cannizzaro ReactionGlenn Vincent Tumimbang100% (7)
- Deamination Lab ReportDocument4 pagesDeamination Lab ReportRyanJForteNo ratings yet
- BaseCatalyzedOxidation ReductionofAldehydesbytheCannizzaroReactionDocument4 pagesBaseCatalyzedOxidation ReductionofAldehydesbytheCannizzaroReactionMike TranNo ratings yet
- CIE Chemistry A Level: 18: Carbonyl CompoundsDocument5 pagesCIE Chemistry A Level: 18: Carbonyl CompoundsayeshaNo ratings yet
- Hydrogenation and Alcohol ClassificationDocument8 pagesHydrogenation and Alcohol ClassificationMuhamad Nazrul BoyoteenNo ratings yet
- Synthesis of An Alkyl HalideDocument4 pagesSynthesis of An Alkyl HalideClyde Co SorianoNo ratings yet
- New Montmorillonite Silylpropylethylenediamine Palladium (II) Complex in Oxidation of Terminal OlefinsDocument6 pagesNew Montmorillonite Silylpropylethylenediamine Palladium (II) Complex in Oxidation of Terminal OlefinsChamula K MasNo ratings yet
- Preparation of IodoformDocument18 pagesPreparation of IodoformHerminHardyantiUtami80% (5)
- Macrocyclic SynthesisDocument3 pagesMacrocyclic SynthesisDenisse Watt CuarterosNo ratings yet
- FR 1 (E6)Document5 pagesFR 1 (E6)JR CastorNo ratings yet
- Artículo de Química Orgánica Laboratorio 11Document3 pagesArtículo de Química Orgánica Laboratorio 11ALDAIR COSSIO POLONo ratings yet
- DEET Insect Repellent Synthesis and AnalysisDocument3 pagesDEET Insect Repellent Synthesis and Analysisnemesisvirus25No ratings yet
- Orgo Lab.Document9 pagesOrgo Lab.ladyjacket42No ratings yet
- Preparation and Purification of An Alkyl Halide FRDocument6 pagesPreparation and Purification of An Alkyl Halide FRCamille GrefaldiaNo ratings yet
- IodoformDocument18 pagesIodoformNurel HidayahNo ratings yet
- Oxidation of Cyclohexanol To CyclohexanoneDocument5 pagesOxidation of Cyclohexanol To CyclohexanoneChandrani Chakraborti100% (1)
- 1Document6 pages170123No ratings yet
- Exer2 PrelabDocument3 pagesExer2 Prelabkarinadegoma100% (1)
- Beta-lactam via Glycol Cleavage and IminationDocument10 pagesBeta-lactam via Glycol Cleavage and IminationMakcaNo ratings yet
- Experiment 2: AROMATIC SIDE-CHAIN OXIDATION: PHTHALIC ACID FROM XYLENEDocument4 pagesExperiment 2: AROMATIC SIDE-CHAIN OXIDATION: PHTHALIC ACID FROM XYLENEPrincess Alyssa Abid0% (1)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Experiment 8Document5 pagesExperiment 8Rohit BiswasNo ratings yet
- Northern Technical University Technical Engineering College/ Mosul Medical Instrumentation Technology EngineeringDocument5 pagesNorthern Technical University Technical Engineering College/ Mosul Medical Instrumentation Technology EngineeringTaha GHNo ratings yet
- CHEM35.1 E2 Aromatic Side Chain OxidationDocument3 pagesCHEM35.1 E2 Aromatic Side Chain OxidationGlenn Vincent TumimbangNo ratings yet
- Preparation and Purification of An Alkyl HalideDocument8 pagesPreparation and Purification of An Alkyl HalideNoOneGotThisUsernameYetNo ratings yet
- Laboratory ReportDocument11 pagesLaboratory ReportElsayed Refaat Aly MareyNo ratings yet
- 12Document6 pages12NathaLie Sta ElenaNo ratings yet
- Sn1 and Sn2 Reactions Write UpDocument6 pagesSn1 and Sn2 Reactions Write UpLevy Medina TrayaNo ratings yet
- Aldol Condensation LabDocument5 pagesAldol Condensation Labnmc515288% (8)
- A. Title of Experiment B. The Aim of ExperimentDocument10 pagesA. Title of Experiment B. The Aim of ExperimentsitrahnurdiniNo ratings yet
- Analytical and Organic ChemistryDocument16 pagesAnalytical and Organic ChemistryNur Ain NadiahNo ratings yet
- Experimen 5 Organic ChemistryDocument8 pagesExperimen 5 Organic ChemistryAbd RaHmanNo ratings yet
- CyclohexeneDocument11 pagesCyclohexeneanon-407590100% (10)
- DehydrationDocument19 pagesDehydrationapi-338215029No ratings yet
- Seperation and Extractions Lab ReportDocument3 pagesSeperation and Extractions Lab ReportDuane HallNo ratings yet
- Synthesis of Cyclohexanol To Cyclohexene - Lab ReportDocument5 pagesSynthesis of Cyclohexanol To Cyclohexene - Lab ReportparisdelapenaNo ratings yet
- Carbonyl Compounds: Properties, Reactions and TestsDocument32 pagesCarbonyl Compounds: Properties, Reactions and TestsYuzamrah Awang NohNo ratings yet
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- An Extraction of SpinachDocument4 pagesAn Extraction of SpinachDenisse Watt Cuarteros67% (3)
- Nitration of Methyl Benzoate (Worksheet)Document1 pageNitration of Methyl Benzoate (Worksheet)Denisse Watt CuarterosNo ratings yet
- Macrocyclic SynthesisDocument3 pagesMacrocyclic SynthesisDenisse Watt CuarterosNo ratings yet
- IR Spectrum For OFF (Mosquito Repellant)Document1 pageIR Spectrum For OFF (Mosquito Repellant)Denisse Watt CuarterosNo ratings yet
- 2,3,4,5 TetraphenylcyclopentadienoneDocument6 pages2,3,4,5 TetraphenylcyclopentadienoneDenisse Watt Cuarteros100% (2)
- NMR For Aldol CondensationDocument1 pageNMR For Aldol CondensationDenisse Watt CuarterosNo ratings yet
- Aldol Condensation DiscussionDocument3 pagesAldol Condensation DiscussionDenisse Watt Cuarteros100% (8)
- Fischer EsterificationDocument3 pagesFischer EsterificationDenisse Watt Cuarteros100% (1)
- Oxone Oxidation Analysis (Borneol and Camphor)Document2 pagesOxone Oxidation Analysis (Borneol and Camphor)Denisse Watt CuarterosNo ratings yet
- Chem 243A - Hydrogenation (Catalytic)Document2 pagesChem 243A - Hydrogenation (Catalytic)Denisse Watt CuarterosNo ratings yet
- Solving The Bacterial UnknownDocument14 pagesSolving The Bacterial UnknownDenisse Watt Cuarteros100% (1)
- Staphylococcus AureusDocument3 pagesStaphylococcus AureusDenisse Watt CuarterosNo ratings yet
- IR Peaks For Cyclic AnhydrideDocument1 pageIR Peaks For Cyclic AnhydrideDenisse Watt CuarterosNo ratings yet
- BCS-CRM 182 - 3 Aug2015Document2 pagesBCS-CRM 182 - 3 Aug2015Ishmael WoolooNo ratings yet
- Resep 1Document44 pagesResep 1Rahadian Noor MadanyNo ratings yet
- Additive 211 PDFDocument4 pagesAdditive 211 PDFChandrakantNo ratings yet
- 9th+class Symbols+and+Formulae Chemistry+MaterialDocument12 pages9th+class Symbols+and+Formulae Chemistry+Materialaveerareddy9No ratings yet
- GROTTHUSS-1805-BOOK-Memoir On The Decomposition of Water ModernDocument5 pagesGROTTHUSS-1805-BOOK-Memoir On The Decomposition of Water Modernglen19No ratings yet
- Ten Test Tube MysteryDocument6 pagesTen Test Tube MysteryCelina CostaNo ratings yet
- Tub & Tile Cleaners - 031Document2 pagesTub & Tile Cleaners - 031mndmattNo ratings yet
- Organic Chemistry Lab Report on Carboxylic AcidsDocument9 pagesOrganic Chemistry Lab Report on Carboxylic AcidsNaimzNaimNo ratings yet
- Analysis of red wine compounds by GC-MSDocument16 pagesAnalysis of red wine compounds by GC-MSNeen NaazNo ratings yet
- 0620 w18 2 1 QPDocument16 pages0620 w18 2 1 QPpNo ratings yet
- F321 Module 3 Practice 5Document4 pagesF321 Module 3 Practice 5coughsyrup123No ratings yet
- Chapter 3 Metals and NonmetalsDocument37 pagesChapter 3 Metals and NonmetalsVibi VibesNo ratings yet
- GENERAL CHEMISTRY 2 - Q1 M3 Concentrations of SolutionDocument16 pagesGENERAL CHEMISTRY 2 - Q1 M3 Concentrations of SolutionJezysaint Ruth Del SocorroNo ratings yet
- FentanylDocument2 pagesFentanylMulayam Singh YadavNo ratings yet
- Chapter 9 Multiple-Choice QuestionsDocument24 pagesChapter 9 Multiple-Choice Questionsteresa tsoiNo ratings yet
- Sr. Chemistry IPE Imp. QuestionsDocument15 pagesSr. Chemistry IPE Imp. Questionssai mukeshNo ratings yet
- Reactivity Series WorksheetDocument6 pagesReactivity Series WorksheetjanithaNo ratings yet
- Closed circuit recovery of copper, lead and iron from electronic waste with citrate solutionsDocument8 pagesClosed circuit recovery of copper, lead and iron from electronic waste with citrate solutionsDemigodNo ratings yet
- Analytical Chemistry Sample Problems SolvedDocument6 pagesAnalytical Chemistry Sample Problems Solvedsantos earlNo ratings yet
- Future Chem Agro Private LimitedDocument2 pagesFuture Chem Agro Private LimitedGarv singhNo ratings yet
- 2 Ethyl HexanolDocument5 pages2 Ethyl Hexanolkallurisurya100% (1)
- Biological Importance of Carbohydrates and LipidsDocument17 pagesBiological Importance of Carbohydrates and LipidsAnaitum SharmaNo ratings yet
- Chemical Technical Data Sheet: Texol™ Propylene Glycol (USP Grade)Document2 pagesChemical Technical Data Sheet: Texol™ Propylene Glycol (USP Grade)Amr RagabNo ratings yet
- BASF Oilfield-Solutions ProductrangeDocument4 pagesBASF Oilfield-Solutions ProductrangePrototype100% (1)
- Bom of Povidone Iodine Solution 5% 2040LtrDocument1 pageBom of Povidone Iodine Solution 5% 2040LtrSujeet KumarNo ratings yet
- Lif .?G, S L L-C-41, J::'': Material SafetyDocument2 pagesLif .?G, S L L-C-41, J::'': Material SafetyRUBEN FERNANDESNo ratings yet
- After-Shower Lotion: UniqemaDocument47 pagesAfter-Shower Lotion: UniqemaadrianNo ratings yet
- Water Impurities & Hardness AnalysisDocument25 pagesWater Impurities & Hardness AnalysisRaviteja VgaNo ratings yet
- AU2020366361A1 - Chlorantraniliprole + BifenthrinDocument92 pagesAU2020366361A1 - Chlorantraniliprole + BifenthrinAlfredo MéndezNo ratings yet
- A Treatise On Phytochemistry by DR Amrit Pal Singh B.SC, B.A.M.S, M.D. (Alternative Medicine)Document166 pagesA Treatise On Phytochemistry by DR Amrit Pal Singh B.SC, B.A.M.S, M.D. (Alternative Medicine)Medhat Sabri100% (1)