Professional Documents
Culture Documents
Risks of Breast Implants
Uploaded by
preciousupset99490 ratings0% found this document useful (0 votes)
32 views10 pagesRisks of Breast Implants
Some of the complications and adverse outcomes of breast imp
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentRisks of Breast Implants
Some of the complications and adverse outcomes of breast imp
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
32 views10 pagesRisks of Breast Implants
Uploaded by
preciousupset9949Risks of Breast Implants
Some of the complications and adverse outcomes of breast imp
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 10
Risks of Breast Implants
Risks of Breast Implants
Some of the complications and adverse outcomes of breast implants include:
Additional surgeries, with or without removal of the device
Capsular contracture, scar tissue that forms around the implant and squeezes the implant
Breast pain
Changes in nipple and breast sensation
Rupture with deflation of saline-filled implants
Rupture with or without symptoms (silent rupture) of silicone gel-filled implants
Implant Complications
The following is a list of local complications and adverse outcomes that occur in at least 1 percent of
breast implant patients at any time. You may need non-surgical treatments or additional surgeries to
treat any of these, and you should discuss any complication and necessary treatment with your
doctor. These complications are listed alphabetically, not in order of how often they occur.
Complication
Description
Asymmetry
The breasts are uneven in appearance in terms of size, shape or breast level.
Breast Pain
Pain in the nipple or breast area
Breast Tissue Atrophy
Thinning and shrinking of the skin
Calcification/Calcium Deposits
Hard lumps under the skin around the implant. These can be mistaken for cancer during
mammography, resulting in additional surgery.
Capsular Contracture
Tightening of the tissue capsule around an implant, resulting in firmness or hardening of the breast
and squeezing of the implant if severe.
Chest Wall Deformity
Chest wall or underlying rib cage appears deformed.
Deflation
Leakage of the saltwater (saline) solution from a saline-filled breast implant, often due to a valve
leak or a tear or cut in the implant shell (rupture), with partial or complete collapse of the implant.
Delayed Wound Healing
Incision site fails to heal normally or takes longer to heal.
Extrusion
The skin breaks down and the implant appears through the skin.
Hematoma
Collection of blood near the surgical site. May cause swelling, bruising and pain. Hematomas
usually occur soon after surgery, but can occur any time there is injury to the breast. The body
may absorb small hematomas, but large ones may require medical intervention, such as surgical
draining.
Iatrogenic Injury/Damage
Injury or damage to tissue or implant as a result of implant surgery
Infection, including Toxic Shock Syndrome
Occurs when wounds are contaminated with microorganisms, such as bacteria or fungi. Most
infections resulting from surgery appear within a few days to a week, but infection is possible any
time after surgery. If an infection does not respond to antibiotics, the implant may need to be
removed
Inflammation/Irritation
Response by the body to an infection or injury. Demonstrated by redness, swelling, warmth, pain
and or/loss of function.
Lymphedema or Lymphadenopathy
Swollen or enlarged lymph nodes
Malposition/Displacement
The implant is not in the correct position in the breast. This can happen during surgery or
afterwards if the implant moves or shifts from its original location. Shifting can be caused by
factors such as gravity, trauma or capsular contracture.
Necrosis
Dead skin or tissue around the breast. Necrosis can be caused by infection, use of steroids in the
surgical breast pocket, smoking, chemotherapy/radiation, and excessive heat or cold therapy.
Nipple/Breast Sensation Changes
An increase or decrease in the feeling in the nipple and/or breast. Can vary in degree and may be
temporary or permanent. May affect sexual response or breast feeding.
Palpability
The implant can be felt through the skin.
Ptosis
Breast sagging that is usually the result of normal aging, pregnancy or weight loss.
Redness/Bruising
Bleeding at the time of surgery can cause the skin to change color. This is an expected symptom due
to surgery, and is likely temporary.
Rupture
A tear or hole in the implant's outer shell.
Seroma
Collection of fluid around the implant. May cause swelling, pain and bruising. The body may
absorb small seromas. Large ones will require a surgical drain.
Skin Rash
A rash on or around the breast.
Unsatisfactory Style/Size
Patient or doctor is not satisfied with the overall look based on the style or size of the implant used.
Visibility
The implant can be seen through the skin.
Wrinkling/Rippling
Wrinkling of the implant that can be felt or seen through the skin.
A complete list of complications, as well as information on rates for those complications can be
found in the patient labeling for the approved breast implants, Labeling for Approved Breast
Implants.
Additional Surgeries
Breast implants are not lifetime devices. The longer you have breast implants, the more likely it is
that complications will occur and you will need to have them removed. There is no guarantee that
you will have a satisfactory cosmetic outcome from any reoperation.
The type of surgical procedure performed during a reoperation depends on the complication
involved. You may need to have one or more reoperations over the course of your life due to one
complication or a combination of local complications. More than one procedure may be performed in
a single reoperation. Types of surgical procedures that may be performed in a reoperation include:
Implant removal, with or without replacement
Capsule removal or surgical release of the scar tissue around the breast implant
Scar or wound revision, such as surgical removal of excess scar tissue
Drainage of a hematoma by inserting a needle or tube through the skin to drain the collection of
blood
Repositioning of the implant by surgically opening the incision and moving the implant
Biopsy/cyst removal by inserting a needle through the skin or cutting through the skin to remove a
lump.
Removal
Removal of the implant(s), with or without replacement, is one type of reoperation. As many as 20
percent of women who receive breast implants for augmentation have to have their implants
removed within 8 to 10 years. You may need to have your implant removed at some time over the
course of your life because of one or more local complications.
After removal, some women do not choose to replace their implants. These women may have
cosmetically undesirable dimpling, puckering, or sagging of their natural breasts.
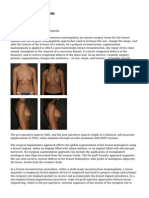
The photograph below shows a 29-year-old woman 1 year after having her silicone gel-filled breast
implants removed, but not replaced. Women with large breast implants, especially those inserted
under and within the breast glands but not on top of the chest muscles (subglandularly), may have
major cosmetic deformity if they choose not to replace them or to undergo additional reconstructive
surgery.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Some insurance companies do not cover implant removal or implant replacement, even if the first
implant surgery was covered.
Capsular Contracture
Capsular contracture is the hardening of the breast around the implant. It can occur in the tissue
surrounding one or both implants. This hardening causes the tissue to tighten, which can be painful.
Capsular contracture may be more common following infection, hematoma and seroma. However,
the cause of capsular contracture is not known.
There are four grades of capsular contracture, known as Baker grades.
Baker Grading Scale
Grade I: Breast is normally soft and looks natural
Grade II: Breast is a little firm but looks normal
Grade III: Breast is firm and looks abnormal
Grade IV: Breast is hard, painful, and looks abnormal
Grades III and IV capsular contracture are considered severe, and may require reoperation. The
surgical procedure breast surgery Pittsburgh usually involves removal of the implant with or without
replacement of the implant. There is a possibility that capsular contracture could occur again after
surgery to correct it.
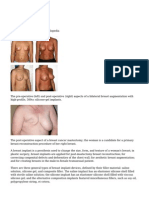
The picture below shows a Grade IV capsular contracture in the right breast of a 29-year- old woman
7 years after placement of silicone gel-filled breast implants.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Rupture
Rupture is a tear or hole in the outer shell of the breast implant. When this occurs in a saline breast
implant, it deflates, meaning the saltwater (saline) solution leaks from the shell. Silicone gel is
thicker than saline, so when a silicone gel-filled implant ruptures, the gel may remain in the shell or
in the scar tissue that forms around the implant (intracapsular rupture). The longer you have a
breast implant, the greater the chance of implant rupture.
The FDA recommends removing both saline-filled and silicone gel-filled breast implants if they have
ruptured. You and your doctor will need to decide whether or not your implant has ruptured and if
you should have it replaced or removed without replacement.
Some possible causes of rupture of breast implants include:
Capsular contracture
Compression during a mammogram
Damage by surgical instruments
Damage during procedures to the breast, such as biopsies and fluid drainage
Normal aging of the implant
Overfilling or underfilling of saline-filled breast implants
Physical stresses such as trauma or intense physical pressure
Placement through a non-FDA approved incision site, for example the belly button
Too much handling during surgery
Rupture and Deflation in Saline-Filled Breast Implants
The term rupture is used for all types of breast implants, but the term deflation is only used for
saline-filled implants. You and/or your doctor will be able to tell if your saline-filled implant ruptures
because the saline solution leaks into your body immediately or over several days. You will notice
that your implant loses its original size or shape.
The following surgical procedures are not recommended for FDA-approved saline-filled breast
implants because they are known to cause rupture and deflation:
Closed capsulotomy - a technique used to relieve capsular contracture involving manually squeezing
the breast to break the hard capsule
Placement of drugs or other substances inside the implant other than sterile saline
Any contact of the implant with Betadine, a povidone-iodine topical antiseptic made by Purdue
Frederick Company
Injection through the implant shell
Alteration of the implant
Stacking of the implants (more than one implant per breast pocket).
The photograph below shows a 30-year-old woman whose left saline-filled breast implant deflated.
The implant is thought to have deflated due to a particular design, which is no longer used by the
manufacturer.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Rupture in Silicone Gel-Filled Implants
If your silicone gel-filled breast implant ruptures, it is not likely that you or your doctor will
immediately notice. Silicone gel is thicker than saline, so when a silicone gel-filled implant ruptures,
the gel may remain in the shell or in the scar tissue that forms around the implant (intracapsular
rupture).
When a silicone gel-filled implant ruptures, a woman may notice a decrease in breast size, change in
breast implant shape, hard lumps over the implant or chest area, an uneven appearance of the
breasts, pain or tenderness, tingling, swelling, numbness, burning, or changes in sensation.
Ruptures that show symptoms usually happen outside of the capsule. However, some ruptures are
called "silent ruptures."
A "silent rupture" doesn't change the way an implant looks or feels to a woman because the rupture
occurs within the capsule. Silent ruptures are not usually evident by a physical examination by a
doctor. Magnetic resonance imaging (MRI) is the most effective method for detecting silent rupture
of silicone gel-filled breast breast plastic surgery Pittsburgh implants. The FDA recommends MRI at
3 years after implantation and every 2 years after that to screen for rupture.
Silicone gel that leaks outside the capsule surrounding the implant may travel (migrate) away from
the breast. The leaked silicone gel may cause lumps to form in the breast or in other tissue, most
often the chest wall, armpit or arm. It may be difficult or impossible to remove silicone gel that has
traveled to other parts of the body.
Connective Tissue Disease
The FDA has not detected any association between silicone gel-filled breast implants and connective
tissue disease, breast cancer, or reproductive problems. In order to rule out these and other rare
complications, studies would need to be larger and longer than these conducted so far.
Breastfeeding
Some women who undergo breast augmentation can successfully breastfeed and some cannot.
Women who undergo mastectomies and then have breast implant reconstruction surgeries may not
be able to breastfeed on the affected side due to loss of breast tissue and the glands that produce
milk.
Effects on Children
At this time, it is not known if a small amount of silicone may pass through from the breast implant
silicone shell into breast milk during breastfeeding. Although there are currently no established
methods for accurately detecting silicone levels in breast milk, a study measuring silicon (one
component in silicone) levels did not indicate higher levels in breast milk from women with silicone
gel-filled implants when compared to women without implants.
In addition, concerns have been raised regarding potential damaging effects on children born to
mothers with implants. Two studies in humans have found no increased risk of birth defects in
children born to mothers who have had breast implant surgery. Although low birth weight, was
reported in a third study, other factors (for example, lower pre-pregnancy weight) may explain this
finding.
Things to Consider Before Getting Breast Implants
(61 KB) (66 KB)
There are several important things to consider before deciding to undergo breast implant surgery,
including understanding your own expectations and reasons for having the surgery. Below are some
things the FDA thinks you should consider before undergoing breast augmentation, reconstruction
or revision surgery.
Breast implants are not lifetime devices; the longer you have your implants, the more likely it will be
for you to have them removed.
The longer you have breast implants, the more likely you are to experience local complications and
adverse outcomes.
The most common local complications and adverse outcomes are capsular contracture, reoperation
and implant removal. Other complications include rupture or deflation, wrinkling, asymmetry,
scarring, pain, and infection at the incision site.
You should assume that you will need to have additional surgeries (reoperations).
Many of the changes to your breast following implantation may be cosmetically undesirable and
irreversible.
If you have your implants removed but not replaced, you may experience changes to your natural
breasts such as dimpling, puckering, wrinkling, breast tissue loss, or other undesirable cosmetic
changes.
If you have breast implants, you will need to monitor your breasts for the rest of your life. If you
notice any abnormal changes in your breasts, you will need to see a doctor promptly.
If you have silicone gel-filled breast implants, you will need to undergo periodic MRI examinations in
order to detect ruptures that do not cause symptoms ("silent ruptures"). For early detection of silent
rupture, the FDA recommends that women with silicone gel-filled breast implants receive MRI
screenings 3 years after they receive a new implant and every 2 years after that. MRI screening for
implant rupture is costly and may not be covered by your insurance.
If you have breast implants, you may have a very low but increased risk of developing a rare type of
cancer called anaplastic large cell lymphoma (ALCL) in the breast tissue surrounding the implant.
ALCL is not breast cancer. Women diagnosed with ALCL in the breast may need to be treated with
surgery, chemotherapy and/or radiation therapy.
You might also like
- Columbia Laboratories Renews Agreement With Merck Serono For CRINONE in Foreign MarketsDocument3 pagesColumbia Laboratories Renews Agreement With Merck Serono For CRINONE in Foreign Marketspreciousupset9949No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- Mentor - Breast ProductsDocument125 pagesMentor - Breast Productsgabbydome4811No ratings yet
- Breast ReductionDocument17 pagesBreast Reductionpreciousupset9949No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationpreciousupset9949No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationpreciousupset9949No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- Breast AugmentationDocument35 pagesBreast Augmentationgabbydome4811No ratings yet
- D 27 A 072 Cbe 7Document30 pagesD 27 A 072 Cbe 7preciousupset9949No ratings yet
- Susan G. Komen® - Understanding Breast Cancer - TreatmentDocument12 pagesSusan G. Komen® - Understanding Breast Cancer - Treatmentpreciousupset9949No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Orbital Implant Peg PlacementDocument510 pagesOrbital Implant Peg PlacementNoorNo ratings yet
- Dissertation MHH HannoverDocument8 pagesDissertation MHH HannoverWriteMySociologyPaperAlbuquerque100% (1)
- F 1108Document4 pagesF 1108Ali AmishNo ratings yet
- AxSOS-ST-30 StrykerDocument20 pagesAxSOS-ST-30 Strykeraditya permadiNo ratings yet
- Biochip & Its Technology: Volume III, Issue VII, July 2014 Ijltemas ISSN 2278 - 2540Document5 pagesBiochip & Its Technology: Volume III, Issue VII, July 2014 Ijltemas ISSN 2278 - 2540Unatti AgarwalNo ratings yet
- Sustainable Biomaterials and Their Applications - A Short ReviewDocument9 pagesSustainable Biomaterials and Their Applications - A Short ReviewvalentinaNo ratings yet
- Anthony Fide's Project Seminar IDocument11 pagesAnthony Fide's Project Seminar ICold LarvaNo ratings yet
- List of BIS Standards As On 07.9.2021Document55 pagesList of BIS Standards As On 07.9.2021Subramani KarurNo ratings yet
- Topic ListDocument3 pagesTopic ListbkprosthoNo ratings yet
- Teknik MRI DasarDocument45 pagesTeknik MRI DasarDeki Kurnia JayaNo ratings yet
- Dupay Synthes BrocherDocument16 pagesDupay Synthes BrocherVISHAKHANo ratings yet
- Eye ProsthesesDocument75 pagesEye ProsthesesNiharika SabharwalNo ratings yet
- Guideline Classification Medical Devices EUDocument26 pagesGuideline Classification Medical Devices EUMehdi100% (1)
- Biomaterials and Implants in Cardiac and Vascular SurgeryDocument9 pagesBiomaterials and Implants in Cardiac and Vascular Surgerytornike kakhetelidzeNo ratings yet
- Introduction to Biomaterials: Materials, Applications and DevelopmentDocument74 pagesIntroduction to Biomaterials: Materials, Applications and Developmentpayaliya91No ratings yet
- EU MDR and IVDR Harmonised StandardsDocument43 pagesEU MDR and IVDR Harmonised Standardskhaled msalbiNo ratings yet
- 11 Soft Tissue Replacement - IDocument39 pages11 Soft Tissue Replacement - IAsina Milas0% (1)
- Dsustrm10161127 3-5mmlcp Clavhkpl TG 150dpiDocument24 pagesDsustrm10161127 3-5mmlcp Clavhkpl TG 150dpiSaifuddin SidikiNo ratings yet
- FDA Guidance Document For Testing Orthopedic Implants - Porous CoatingsDocument10 pagesFDA Guidance Document For Testing Orthopedic Implants - Porous Coatingsmaven3No ratings yet
- Azevedo, 2002 (Engineering Failure Analysis, Falha em Implantes No Brasil)Document13 pagesAzevedo, 2002 (Engineering Failure Analysis, Falha em Implantes No Brasil)Sócrates NajarNo ratings yet
- J Matdes 2016 09 029Document8 pagesJ Matdes 2016 09 029GUSTAVO LOPEZ MENDOZANo ratings yet
- BM2303 - BIOMATERIALS EXAM REVIEWDocument10 pagesBM2303 - BIOMATERIALS EXAM REVIEWMythily VedhagiriNo ratings yet
- International Journalof Chemical Nuclear Metallurgicaland Materials EngineeringDocument7 pagesInternational Journalof Chemical Nuclear Metallurgicaland Materials EngineeringMohammed GhisheerNo ratings yet
- NuEra Tight 1.2 - 8 Pages - US - PRESS - PB-00018880US-A Rev ADocument8 pagesNuEra Tight 1.2 - 8 Pages - US - PRESS - PB-00018880US-A Rev ABiomed Medex OmanNo ratings yet
- MRI Safety Screening Form - Amended - 30NOV2016Document1 pageMRI Safety Screening Form - Amended - 30NOV2016FC LOONNo ratings yet
- Current Concepts in Orthopaedic Biomaterials and Implant FixationDocument24 pagesCurrent Concepts in Orthopaedic Biomaterials and Implant FixationS.ENo ratings yet
- MR Safe Conditional PDFDocument4 pagesMR Safe Conditional PDFAKSNo ratings yet
- CPT Business Report 10272020Document9 pagesCPT Business Report 10272020api-549352158No ratings yet
- F366Document5 pagesF366Gustavo SuarezNo ratings yet
- PJIsDocument29 pagesPJIsMohammed Alfahal100% (1)