Professional Documents
Culture Documents
Eye Care Nursing Center
Uploaded by
Juliana Borges MoraisOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Eye Care Nursing Center
Uploaded by
Juliana Borges MoraisCopyright:
Available Formats
ajn@lww.com AJN January 2006 Vol. 106, No.
1 72AA
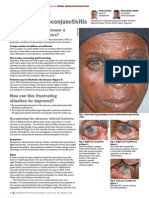
Eye Care for Patients in the ICU
with muscle relaxants to facili-
tate care. Other patients may be
unconscious as a result of an
underlying medical condition
such as a head injury. Normal
eyelid closure is maintained dur-
ing sleep by the tonic contrac-
tion of the orbicularis oculi
muscle. The use of muscle relax-
ants reduces that contraction
and often results in the need for
passive eyelid closure.
Additionally, sedation may
result in a lack of random eye
movements and a loss of the
blink reflex. These factors inter-
fere with tear film coverage of
the eye. Inadequate eyelid clo-
sure permits an increase in tear
film evaporation. As a result,
patients become susceptible to
dessication (drying) of the eye.
This can be exacerbated by a
decrease in secretions caused by
medications such as atropine,
antihistamines, phenothiazines,
disopyramide (Norpace), and tri-
cyclic antidepressants. These fac-
tors seriously impair corneal and
conjunctival surface defenses.
POTENTIAL OPHTHALMIC PROBLEMS
Exposure and drying of the eye
can result in superficial ker-
atopathy, a noninflammatory
disease of the cornea. The
epithelial surface of the cornea is
compromised, and the resultant
corneal exposure can lead to
ulceration, perforation, and scar-
ring, which, though usually self-
limiting, may cause permanent
damage. Superficial corneal
abrasions are a more common
result of eye exposure.
According to a systematic liter-
ature review,
1
there are indica-
tions that corneal abrasions can
occur in ICU patients within a
relatively short time, ranging
from 48 hours to one week. In
three randomized controlled tri-
als, the incidence of such abra-
sions in this population ranged
from 3.33% to 22%. In addition,
one prospective analysis of 50
randomly selected intensive care
patients found that 40% had
corneal abrasions. Another study
suggested that up to 60% of ICU
patients who receive sedation for
longer than 48 hours will develop
them. And still another study
reported that corneal abrasions
were detected in 42% of ICU
patients, most within the first
week of admission.
Any inflammation of the
cornea (keratitis) increases the
risk of infection. Bacterial kerati-
tis is considered a dire complica-
tion associated with corneal
exposure and a compromise in
the normal tear film. Conjunct-
ivitis, which may be caused by
bacterial or viral infection,
allergy, or environmental factors,
is another risk.
Conjunctival edema (chemo-
sis; also called ventilator eye)
is viewed as the result of the
adverse effects of both ventila-
tory support and the drugs used
to facilitate it. These can generate
an acute increase in intraocular
pressure, which promotes sub-
conjunctival hemorrhage. Ocular
problems associated with ventila-
tion are thought to occur with
high intrathoracic pressure and,
Best Practice is a new column from the Joanna
Briggs Institute (JBI), Adelaide, South
Australia (www.joannabriggs.edu.au), that
will appear periodically in AJN. This install-
ment is based upon a systematic literature
review conducted by Nicole Joyce under the
supervision of JBI. It is adapted with permis-
sion from the original text by Nicole Joyce
and David Evans, PhD, that appeared in Best
Practice 2002;6(1):1-6. While care has been
taken to ensure that this article summarizes
available research and expert consensus, the
Institute has not conducted a formal update
since the original publication, and no liability
can be accepted as a result of reliance on the
procedures described. [Editors note: An intro-
duction to Best Practice and JBI appears on
page 82.]
CRITICAL CARE EXTRA
A
ll patients in acute
care settings who
have impaired or
compromised pro-
tective mechanisms
are at risk for eye infection or
injury during their stay. Those
who are unconscious, sedated,
or paralyzed are at especially
high risk.
The incidence of eye disorders
in the intensive care population
is difficult to quantify. Factors
may include poor documenta-
tion and the fact that eye care is
often seen as a relatively minor
concern when the patient is criti-
cally ill. Eye complications can
range from mild conjunctival
infection to serious corneal
injury. Permanent ocular damage
may result from ulceration, per-
foration, vascularization, and
scarring of the cornea.
LOSS OF PROTECTIVE MECHANISMS
People admitted to ICUs often
require mechanical ventilation,
and the majority of these
patients are sedated to ensure
comfort and facilitate treatment.
Some patients may be paralyzed
t
conjunctiva prolapse beyond the
eyelids, this can cause corneal
drying and defective epithelial
repair.
The literature search found no
studies that had investigated
interventions to prevent conjucti-
val edema or infection. The lack
of research in this area makes
determination of both the scope
of these problems and the effec-
tiveness of treatments difficult.
REVIEW FINDINGS
Eye care procedures often vary
considerably from one institu-
tion to another. Interventions
may be grouped into four cate-
gories: eye hygiene, preventing
dry eyes, ensuring eyelid closure,
and programs of eye care.
The literature search identi-
fied six clinical trials that evalu-
ated the effectiveness of eye care
interventions: three randomized
controlled trials, one controlled
trial, one uncontrolled trial, and
a before-and-after study. It
should be noted that results
from the three randomized con-
trolled trials are relevant in more
than one intervention category,
as these trials evaluated a range
of interventions.
Eye hygiene. Cleansing meth-
ods typically included the use of
sterile packs containing sterile
water, cotton balls, and a gallipot
(a small vessel to hold solution);
irrigation with normal saline; or
gauze soaked in normal saline or
sterile water. Despite many sug-
gestions in the literature regard-
ing hygiene regimens or regular
cleansing of the eye, no research
was identified evaluating this
aspect of eye care.
Preventing dry eyes. A vari-
ety of approaches have been used
to maintain the tear film and pre-
vent corneal dessication, including:
moisture chambers created by
using polyethylene film to
cover the eye covers)
methylcellulose drops
methylcellulose ointment
general lubricants
polyacrylamide gel
paraffin gauze dressing
hypromellose drops (artificial
tears)
lubricating prophylactic
antibiotics
Two randomized controlled
trials were identified that evalu-
ated methods to prevent the dry-
ing of the ocular surface. One
trial investigated the effectiveness
of eye drops, the other, eye oint-
ments. The effectiveness of these
two interventions was measured
in terms of their ability to pre-
vent corneal abrasions.
Eye drops. A single random-
ized controlled trial compared
the effectiveness of regular instil-
lations of methylcellulose drops
(Methopt Forte) to that of poly-
ethylene film covers (Gladwrap)
over the eyes. The polyethylene
film group had significantly
fewer corneal abrasions (one
person out of 30) than did the
methylcellulose drops group
(eight people out of 30).
Eye ointments. Two random-
ized controlled trials examined
the instillation of ointments for
the prevention of corneal abra-
sions. One trial compared the
effectiveness of artificial-tear
ointment (Duratears) to that of
passive eyelid closure. Passive
eyelid closure in this trial con-
in particular, with the use of posi-
tive end-expiratory pressure
(PEEP) of 5 cm H
2
O or higher.
Exacerbation of conjunctival
edema has been said to occur if
the tape securing the endotra-
cheal tube is too tight. This can
compromise venous return from
the head, leading to venous con-
gestion and potentially increasing
intraocular pressure. Conjunc-
tival edema may lead to inade-
quate eyelid closure; if the
72BB AJN January 2006 Vol. 106, No. 1 http://www.nursingcenter.com
CRITICAL CARE EXTRA
Levels of Evidence
All studies were categorized
according to the strength of the evi-
dence, based on the following revised
classification system.
2
Level 1: The evidence was obtained
from a systematic review of all rele-
vant randomized controlled trials.
Level 2: The evidence was obtained
from at least one properly designed
randomized controlled trial.
Level 3.1: The evidence was
obtained from well-designed,
pseudorandomized (randomized by
alternate allocation or other
method), controlled trials.
Level 3.2: The evidence was
obtained from cohort studies with
concurrent controls and nonrandom-
ized allocation, case-control stud-
ies, or interrupted time (multiple
observations over time) series with
a control group.
Level 3.3: The evidence was
obtained from comparative studies
with historical controls, two or more
single-arm studies, or interrupted
time series without a control group.
Level 4: The evidence was obtained
from case series, either posttest or
pretest and posttest.
t
instilled products in reducing
corneal abrasions.
Ensuring eyelid closure. Num-
erous approaches have been used
to ensure eyelid closure, including
adhesive tape, treated gauze, eye
patches, and temporary sutures,
but none have yet been evaluated.
However, one study found that
passive eyelid closure performed
by nurses was significantly less
effective at preventing corneal
abrasions than was the use of arti-
ficial tear ointment (Duratears).
Eye care programs. The liter-
ature suggests that attempts have
been made to standardize eye
care in ICUs through education
of staff, development and imple-
mentation of eye care algo-
rithms, and development of
general guidelines for eye care.
However, although specific pro-
grams of eye care have been pro-
posed in the literature, none
have been evaluated by random-
ized controlled trials.
Impact on the family. One
paper noted that placing
Gladwrap over the eyes of a
patient in the ICU had a signifi-
cant impact on the patients
appearance; anecdotal informa-
tion suggests that this and other
sisted of the nurse physically
closing the eyelids. Fewer corneal
abrasions occurred in the artifi-
cial-tear ointment group (two
people out of 25) than occurred
in the passive eyelid closure
group (nine people out of 25).
The other trial examined the
effectiveness of hypromellose
ointment (Lacrilube) applied
every two hours to that of poly-
ethylene film covers. The results
suggested that there was no sig-
nificant difference in the inci-
dence of corneal abrasions
between the hypromellose oint-
ment group (four people out of
60) and the polyethylene film
group (none out of 50).
Polyethylene film covers. Two
randomized controlled trials
evaluated the use of polyethylene
film covers as a measure to pre-
vent dry eyes. Both trials com-
pared the efficacy of polyeth-
ylene film covers to that of
instilled eye products (methylcel-
lulose drops in one trial and
hypromellose ointment [Lacri-
lube] in the other). The findings
were then pooled in a meta-
analysis, which demonstrated a
significant difference in favor of
polyethylene film covers over
Polyethylene film covers
are more effective at reducing
the incidence of corneal abrasions
than are ointments and drops.
eye care interventions may also
have significant effects on family
members. However, this issue
has not yet been investigated.
RECOMMENDATIONS
In summary, although many eye
care interventions and products
are used, or are recommended for
use in the literature, few have
been evaluated in the intensive
care setting. The following recom-
mendations are based on the find-
ings of three small randomized,
controlled trials (level 2 evidence):
Eye care should be part of the
care provided to all people
upon admission to ICUs.
Polyethylene film covers are
more effective at reducing the
incidence of corneal abrasions
than are ointments and drops.
Ointments and drops are
more effective at reducing the
incidence of corneal abrasions
than is no eye instillation.
Further research is urgently
needed.
For more information, con-
tact the Joanna Briggs
Institute, Level 4, Margaret
Graham Building, Royal
Adelaide Hospital, Adelaide
5000, SA, Australia; +61-8-
8303-4880; or visit www.
joannabriggs.edu.au.
REFERENCES
1. Joyce N. Eye care for the intensive
care patienta systematic review.
Adelaide, Australia: The Joanna Briggs
Institute for Evidence Based Nursing
and Midwifery; 2002. No. 21.
2. National Health and Medical
Research Council. A guide to the
development, evaluation and imple-
mentation of clinical practice guide-
lines. Canberra, Australia: The
Council; 1999: http://www.nhmrc.
gov.au/publications/_files/cp30.pdf.
CRITICAL CARE EXTRA t
72DD AJN January 2006 Vol. 106, No. 1 http://www.nursingcenter.com
You might also like
- Clinical StudyDocument7 pagesClinical StudyAndre AzharNo ratings yet
- Clinical Management Review 2023-2024: Volume 2: USMLE Step 3 and COMLEX-USA Level 3From EverandClinical Management Review 2023-2024: Volume 2: USMLE Step 3 and COMLEX-USA Level 3No ratings yet
- Dry Eye ThesisDocument5 pagesDry Eye ThesisPaperWritingServiceCollegeUK100% (2)
- Blepharitis: A Comprehensive Clinical GuideFrom EverandBlepharitis: A Comprehensive Clinical GuideAsim V. FarooqNo ratings yet
- Comparison of A Preservative Free Nonsteroidal.Document7 pagesComparison of A Preservative Free Nonsteroidal.Danty IndriastutyNo ratings yet
- Adult Critical Care Eye Care GuidelineDocument19 pagesAdult Critical Care Eye Care GuidelineLuffy Jemari Luffy JemariNo ratings yet
- JurnalDocument8 pagesJurnalAnonymous fgRHAEIMrHNo ratings yet
- JurnalDocument18 pagesJurnalJean UsmanyNo ratings yet
- Dry Eye Disease GuideDocument25 pagesDry Eye Disease Guide215045 zulfa laili aNo ratings yet
- Hydroxypropyl Cellulose Ophthalmic Inserts (Lacrisert) Reduce The Signs and Symptoms of Dry Eye Syndrome and Improve Patient Quality of LifeDocument9 pagesHydroxypropyl Cellulose Ophthalmic Inserts (Lacrisert) Reduce The Signs and Symptoms of Dry Eye Syndrome and Improve Patient Quality of LifekikinantiNo ratings yet
- Cyclosporine (0.05%) Combined With Diclofenac Sodium EyeDocument6 pagesCyclosporine (0.05%) Combined With Diclofenac Sodium EyeelfiahNo ratings yet
- Dry Eye ManagementDocument8 pagesDry Eye ManagementMul YaniNo ratings yet
- Trends in The Therapeutic Drug Delivery System For The Management of Glaucoma A ReviewDocument8 pagesTrends in The Therapeutic Drug Delivery System For The Management of Glaucoma A ReviewInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Special EmergenciesDocument152 pagesSpecial EmergenciesJannell LawesNo ratings yet
- Disturbance in Sensory PerceptionDocument48 pagesDisturbance in Sensory PerceptionKristine Louise JavierNo ratings yet
- Jogi - Basic Ophthalmology, 4th Edition (Ussama Maqbool)Document7 pagesJogi - Basic Ophthalmology, 4th Edition (Ussama Maqbool)pipikafiyaNo ratings yet
- Evaluation of The Painful EyeDocument9 pagesEvaluation of The Painful EyeMuthia Farah AshmaNo ratings yet
- Tear Film, Contact Lens, and Patient-Related Factors Associated With Contact Lens-Related Dry EyeDocument10 pagesTear Film, Contact Lens, and Patient-Related Factors Associated With Contact Lens-Related Dry EyeAlda Gitu RahmaNo ratings yet
- Retina 2012 Sep 32 (Suppl 2) S225-31Document7 pagesRetina 2012 Sep 32 (Suppl 2) S225-31roro207No ratings yet
- Guidelines For The Management of Corneal Ulcer WHODocument36 pagesGuidelines For The Management of Corneal Ulcer WHOichalledhaNo ratings yet
- CMC - Tear Film StabilityDocument10 pagesCMC - Tear Film StabilityTushar BatraNo ratings yet
- Non-Surgical Management of Hyphaema Among Nigerian OphthalmologistsDocument5 pagesNon-Surgical Management of Hyphaema Among Nigerian OphthalmologistsKaisun TeoNo ratings yet
- Topical Tacrolimus For Corneal Subepithelial InfiltratesDocument4 pagesTopical Tacrolimus For Corneal Subepithelial InfiltratessrihandayaniakbarNo ratings yet
- Iritis 8Document14 pagesIritis 8Alvis KurniawanNo ratings yet
- Tgs ReferatDocument44 pagesTgs ReferatlieNo ratings yet
- Glaucoma and CataractDocument30 pagesGlaucoma and CataractJayselle ArvieNo ratings yet
- New 5-FU option for epithelial downgrowthDocument4 pagesNew 5-FU option for epithelial downgrowthDelila MaharaniNo ratings yet
- Ruptur BulbiDocument5 pagesRuptur BulbiseptrealtiNo ratings yet
- Management of Glaucoma Medication Induced Dry Eye DiseaseDocument7 pagesManagement of Glaucoma Medication Induced Dry Eye DiseaseMeilin ZulfatinNo ratings yet
- Vitrectomy in Endophthalmitis: Kapil Bhatia, Avinash Pathengay and Manav KheraDocument17 pagesVitrectomy in Endophthalmitis: Kapil Bhatia, Avinash Pathengay and Manav KheraGrady ChristianNo ratings yet
- Dry Eye Disease after LASIK SurgeryDocument3 pagesDry Eye Disease after LASIK SurgeryRatih Kusuma DewiNo ratings yet
- Jurnal MataDocument11 pagesJurnal MataNovi RatnaNo ratings yet
- Thesis Dry EyeDocument7 pagesThesis Dry Eyebsfp46eh100% (2)
- Dry Eye Syndrome - StatPearls - NCBI Bookshelf PDFDocument7 pagesDry Eye Syndrome - StatPearls - NCBI Bookshelf PDFnum padNo ratings yet
- Treating Epithelial Downgrowth with 5-Fluorouracil InjectionsDocument5 pagesTreating Epithelial Downgrowth with 5-Fluorouracil Injectionsaghnia jolandaNo ratings yet
- Another JurnalDocument4 pagesAnother JurnalReskyAmeliaHRNo ratings yet
- False Myths Versus Medical Facts: Ten Common Misconceptions Related To Dry Eye DiseaseDocument10 pagesFalse Myths Versus Medical Facts: Ten Common Misconceptions Related To Dry Eye DiseaseEduardo PiresNo ratings yet
- The Course of Dry Eye After Phacoemulsification Surgery: Researcharticle Open AccessDocument5 pagesThe Course of Dry Eye After Phacoemulsification Surgery: Researcharticle Open AccessDestia AnandaNo ratings yet
- Comparative Evaluation of Dry Eye Following Cataract Surgery: A Study From North IndiaDocument6 pagesComparative Evaluation of Dry Eye Following Cataract Surgery: A Study From North IndiaInternational Organization of Scientific Research (IOSR)No ratings yet
- Surgical Management of Anterior UveitisDocument3 pagesSurgical Management of Anterior UveitisTri BasukiNo ratings yet
- Rematoid AtritisDocument9 pagesRematoid AtritisNovelis TriwikarnoNo ratings yet
- Jovr 6 3 192Document8 pagesJovr 6 3 192Felicia JesslynNo ratings yet
- Contact Lens Complications - EyeWikiDocument2 pagesContact Lens Complications - EyeWikiSania NadianisaNo ratings yet
- Vitreous Floaters: Publication DetailsDocument6 pagesVitreous Floaters: Publication Detailsmithaa octoviagnesNo ratings yet
- (Osborn) Chapter 71: Learning Outcomes (Number and Title)Document16 pages(Osborn) Chapter 71: Learning Outcomes (Number and Title)KittiesNo ratings yet
- Corneal Edema Management With Ededay in Post CataractDocument12 pagesCorneal Edema Management With Ededay in Post CataractRamon Andres SrNo ratings yet
- Guide to Managing Microbial KeratitisDocument30 pagesGuide to Managing Microbial KeratitisTatik Handayani100% (1)
- Opth 8 581 OsmolaridadDocument10 pagesOpth 8 581 Osmolaridadmono1144No ratings yet
- Vernal Keratoconjunctivitis: Why Is Allergic Eye Disease A Problem For Eye Workers?Document3 pagesVernal Keratoconjunctivitis: Why Is Allergic Eye Disease A Problem For Eye Workers?darendraabimayuNo ratings yet
- Cycloplegic Refraction in Optometric Practice 1337594763401 2Document14 pagesCycloplegic Refraction in Optometric Practice 1337594763401 2Strauss de LangeNo ratings yet
- MataDocument16 pagesMataIslah NadilaNo ratings yet
- 1 s2.0 S1367048416000059 MainDocument3 pages1 s2.0 S1367048416000059 MainArturo GuilliemNo ratings yet
- Evaluation of Umbilical Cord Serum Therapy in Acute Ocular Chemical BurnsDocument6 pagesEvaluation of Umbilical Cord Serum Therapy in Acute Ocular Chemical BurnsDebby Astasya AnnisaNo ratings yet
- Jovr 6 3 192 PDFDocument7 pagesJovr 6 3 192 PDFShofura AzizahNo ratings yet
- Folded Bandage Contact Lens Retention in A Patient With Bilateral Dry Eye SymptomsDocument85 pagesFolded Bandage Contact Lens Retention in A Patient With Bilateral Dry Eye SymptomsAgung Putra EvashaNo ratings yet
- Jurnal Mata Sodium Hyaluronate PDFDocument7 pagesJurnal Mata Sodium Hyaluronate PDFAstrid RumbiaNo ratings yet
- Comparison of Ketorolac 0.4 and Nepafenac 0.1 For The Prevention of Cystoid Macular Oedema After PhacoemulsifiDocument6 pagesComparison of Ketorolac 0.4 and Nepafenac 0.1 For The Prevention of Cystoid Macular Oedema After PhacoemulsifiMinh NguyenNo ratings yet
- Management and Diagnosis of Viral, Bacterial, and Allergic ConjunctivitisDocument30 pagesManagement and Diagnosis of Viral, Bacterial, and Allergic ConjunctivitisAnonymous yyR4FGNo ratings yet
- ICU Eye Care Guidelines for Preventing DiseaseDocument6 pagesICU Eye Care Guidelines for Preventing DiseaseKiki dwi PratiwiNo ratings yet
- French and American FeminismDocument21 pagesFrench and American FeminismJuliana Borges MoraisNo ratings yet
- Single Effect PoeDocument2 pagesSingle Effect PoeLina JacovkisNo ratings yet
- Trabalhando Com A SombraDocument13 pagesTrabalhando Com A SombraJuliana Borges MoraisNo ratings yet
- Sentido Da CriseDocument14 pagesSentido Da CriseJuliana Borges MoraisNo ratings yet
- In Sync 3Document73 pagesIn Sync 3Juliana Borges MoraisNo ratings yet
- Close Reading Short StoriesDocument5 pagesClose Reading Short StoriesJuliana Borges MoraisNo ratings yet
- Sucking Salt Caribbean Women Writers Migration and SurvivalDocument241 pagesSucking Salt Caribbean Women Writers Migration and SurvivalJuliana Borges Morais100% (1)
- Writing Love Story Ok2Document2 pagesWriting Love Story Ok2Juliana Borges MoraisNo ratings yet
- J Gray Testing and Evaluation Instruments Research OrganizerDocument5 pagesJ Gray Testing and Evaluation Instruments Research Organizerapi-528983071No ratings yet
- Shapiro-Wilk TestDocument3 pagesShapiro-Wilk Testdev414No ratings yet
- The Objective of Selection Decision Is ToDocument20 pagesThe Objective of Selection Decision Is Toskumar002100% (5)
- Writing A Position Paper Writing A Position PaperDocument5 pagesWriting A Position Paper Writing A Position PaperDavid Gallano100% (1)
- iX6Iazv1Rfq-biostatistics MineDocument6 pagesiX6Iazv1Rfq-biostatistics MineShashank AlokNo ratings yet
- Spiritualism, Mesmerism and The Occult 1800-1920 Colour LeafletDocument4 pagesSpiritualism, Mesmerism and The Occult 1800-1920 Colour LeafletPickering and ChattoNo ratings yet
- Chapter2: Research MethodologyDocument2 pagesChapter2: Research MethodologyAlex KaminjaNo ratings yet
- Very Important Notes For PMI-ACP ExamDocument18 pagesVery Important Notes For PMI-ACP Examdarff45100% (10)
- Well Integrity Risk Assessment in Geothermal Wells - Status of TodayDocument93 pagesWell Integrity Risk Assessment in Geothermal Wells - Status of Todaymayalasan1No ratings yet
- WINGS OF FIRE: KALAM'S JOURNEYDocument24 pagesWINGS OF FIRE: KALAM'S JOURNEYHacking Something100% (1)
- Parametros Fuerza ChileDocument11 pagesParametros Fuerza ChileCinthya CórdovaNo ratings yet
- Final ProposalDocument5 pagesFinal Proposalapi-550445595No ratings yet
- ARI Varmam Therapy Mail Today Article 25jan 2011Document1 pageARI Varmam Therapy Mail Today Article 25jan 2011Arts Research InstituteNo ratings yet
- Zamboanga Del Sur Provincial Government CollegeDocument43 pagesZamboanga Del Sur Provincial Government Collegeklient rebuyonNo ratings yet
- Online Testing Methods and FormatsDocument75 pagesOnline Testing Methods and FormatsyaswanthNo ratings yet
- CRP point-of-care testing implementation in nursing homes evaluatedDocument11 pagesCRP point-of-care testing implementation in nursing homes evaluatedPuttryNo ratings yet
- Purpose of CalibrationDocument2 pagesPurpose of Calibrationsakata_abera4No ratings yet
- SSRN Id3607845 PDFDocument39 pagesSSRN Id3607845 PDFSandman CodNo ratings yet
- IDT Module 1Document22 pagesIDT Module 1adarshamrajannaNo ratings yet
- Unit 2 Project Identification and SelectionDocument30 pagesUnit 2 Project Identification and SelectionDeepak Rao RaoNo ratings yet
- Theoretical FrameworkDocument3 pagesTheoretical FrameworkAngelo Marcaida LLanes100% (2)
- Stuart Hall School Spring Newsletter 2010Document8 pagesStuart Hall School Spring Newsletter 2010StuartHallSchoolNo ratings yet
- Vietnam's National Foreign Language 2020 ProjetDocument3 pagesVietnam's National Foreign Language 2020 ProjetPhạm Tài100% (1)
- A Performance Comparison of Multi-Objective Optimization-BasedDocument13 pagesA Performance Comparison of Multi-Objective Optimization-Basedmeysam_gholampoorNo ratings yet
- Boosting-Performance-Through-Organization-DesignDocument7 pagesBoosting-Performance-Through-Organization-DesignAyoub AroucheNo ratings yet
- RGPV Act EngDocument39 pagesRGPV Act EngVikas Chandra100% (1)
- Poshandevi Social Studies SBA COMPLETEDDocument23 pagesPoshandevi Social Studies SBA COMPLETEDFrëßh AsíáñNo ratings yet
- Secondary: Qualitative ResearchDocument5 pagesSecondary: Qualitative ResearchFISB_TaniyaNo ratings yet
- Screenshot 2022-08-30 at 19.22.45Document57 pagesScreenshot 2022-08-30 at 19.22.45Garn Supratid2022No ratings yet
- Plant Maintenance Budget and Cost AnalysisDocument31 pagesPlant Maintenance Budget and Cost AnalysisWhafims100% (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (402)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (4)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (13)
- The Happiness Trap: How to Stop Struggling and Start LivingFrom EverandThe Happiness Trap: How to Stop Struggling and Start LivingRating: 4 out of 5 stars4/5 (1)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 5 out of 5 stars5/5 (3)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsNo ratings yet
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- The Ultimate Guide To Memory Improvement TechniquesFrom EverandThe Ultimate Guide To Memory Improvement TechniquesRating: 5 out of 5 stars5/5 (34)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsFrom EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNo ratings yet
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (31)
- Techniques Exercises And Tricks For Memory ImprovementFrom EverandTechniques Exercises And Tricks For Memory ImprovementRating: 4.5 out of 5 stars4.5/5 (40)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisFrom EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisRating: 5 out of 5 stars5/5 (3)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeFrom EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeRating: 4.5 out of 5 stars4.5/5 (253)
- Secure Love: Create a Relationship That Lasts a LifetimeFrom EverandSecure Love: Create a Relationship That Lasts a LifetimeRating: 5 out of 5 stars5/5 (17)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (327)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)