Professional Documents
Culture Documents
HSC Chemistry CH 1 Production of Materials Revision Notes Student
Uploaded by
Wing ChuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HSC Chemistry CH 1 Production of Materials Revision Notes Student
Uploaded by
Wing ChuCopyright:
Available Formats
9.2 Production of materials: 1.
Fossil fuel products
1. Fossil fuels
provide both
energy and raw
materials such
as ethylene, for
the production
of other
substances
Students learn to:
construct word and balanced
formulae equations of
chemical reactions as they
are encountered
identify the industrial source
of ethylene from the cracking
of some of the fractions from
the refining of petroleum
identify that ethylene,
because of the high reactivity
of its double bond, is readily
transformed into many useful
products
identify that ethylene serves
as a monomer from which
polymers are made
identify polyethylene as an
addition polymer and explain
the meaning of this term
outline the steps in the
production of polyethylene as
an example of a commercially
and industrially important
polymer
identify the following as
commercially significant
monomers:
o vinyl chloride
o styrene
o by both their
systematic and
common names
describe the uses of the
polymers made from the
above monomers in terms of
their properties
Students:
gather and present
information from first-hand or
secondary sources to write
equations to represent all
chemical reactions
encountered in the HSC
course
identify data, plan and
perform a first-hand
investigation to compare the
reactivities of appropriate
alkenes with the
corresponding alkanes in
bromine water
analyse information from
secondary sources such as
computer simulations,
molecular model kits or
multimedia resources to
model the polymerisation
process
Ch1 Production of materials/ Revision notes / p. 1
Alkene + bromine water
e.g.
Word equation propene + bromine water
Chemical
equation
+ Br
!aq"
#bservable changes$ %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
&'planation$ %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
Alkane + bromine water
(o reaction
Production of polyethylene from natural raw material :
%%%%%%%%%%%%%%%%%%% is e'tracted from subterranean deposits and separated into component
h)drocarbon molecules using %%%%%%%%%%%%%%%%%%%%%%%%%%%%. * portion of the higher boiling point
fractions are converted to eth)lene in a chemical process called %%%%%%%%%%%%%%%%%+ whereb) these
larger molecules are bro,en into smaller ones b) using %%%%%%%%%%% and/or pressure.
crude oil components of h)drocarbon molecules - fractional distillation
C
1.
/
0
C
11
/
+ C
.
/
11
- crac,ing
C
.
/
11
C
0
/
2
+ C
/
3
!eth)lene"
&th)lene is used b) the petrochemical industr) as a monomer substrate for the production of a number of
different %%%%%%%%%%%%%% !long chain molecules made up of repeating monomer units". 4an) different
t)pes of plastic bottles are manufactured from one such pol)mer %%%%%%%%%%%%%%%%%%%%%%. 5his
molecule is formed when man) eth)lene monomer units chemicall) 6oin b) 7opening out8 their
%%%%%%%%%%% bonds to form single bonds between neighbouring units+ without the loss of an) atoms.
5herefore pol)eth)lene is ,nown as an %%%%%%%%%%%%%%%%%%% pol)mer. 5he following equation
describes this pol)merisation process.
C/
- C/
+ C/
- C/
+ C/
- C/
C/
C/
C/
C/
C/
C/
5he pol)merisation of eth)lene as an industrial process is carried out using one of two methods+ both of
which involve a form of chemical initiation and termination.
5he older gas phase results in significant chain branching between pol)eth)lene molecules resulting in a
%%%%%%%%%%9densit) pol)eth)lene product. :t is relativel) soft and is well suited for the production of soft
plastic bottles li,e mil, and personal drin,ing bottles.
5he newer ;iegler < (atta process forms %%%%%%%%%%%%%%% pol)eth)lene molecules resulting in a harder
tougher %%%%%%%%%%%%% densit) pol)eth)lene product+ which can be used for plastic bottles that need to
be harder and more durable.
Ch1 Production of materials/ Revision notes / p.
Polyethylene is produced by addition polymerisation. The following graph shows the distribution of
moleuvular weight of polymer molecules in the sample:
5ermination of the pol)merisation process occurs
%%%%%%%%%%%%%%%%% at different stages for different
molecules in the reaction mi'ture. Pol)mers of different
lengths and hence different molecular weight are produced.
5he reaction conditions influences the average chain length.
Ch1 Production of materials/ Revision notes / p. 0
4onomer Pol)mer =ses
>tructural formula Common
name
>)stemic name
&th)lene
Cling9wrap film+
?le'ible food bag
@arbage bin+ rigid
plastic to)
Ain)l
chloride
!PAC"
&lectric wire
coating+ water
pipe
>t)rene Bisposable foam
drin,ing cup+
pac,aging of
fragile devices
Biopolymer should be used to replace plastic
Cong9lasting+ non9%%%%%%degradable pol)mers such as pol)st)rene greatl) affect the environment+ as
microorganisms cannot brea, them down. Pol)st)rene is lightweight and can be made into a solidif)ing
foam to be used in insulating cups. :t can also be manufactured into a clear+ hard and brittle plastic for
drin,ing cups. 5hese properties ma,e pol)st)rene a useful pol)mer. /owever+ due to its inabilit) to
biodegrade and most of its uses being short9lived+ pol)st)rene debris builds up in the environment.
Pol)lactic acid !PC*" is an alternative to traditional pol)mers. PC* is classed as a %%%%%%%%%%%%%%%%%
as it is biodegradable. :t is used for short9lived applications such as cold/warm drin,ing cups and plastic
bags. *s PC* is made from the waste products of corn crops+ which are converted b) bacteria into lactic
acid and then reacted to form PC*+ it is easil) bro,en down b) microorganisms once it has been disposed
of.
5herefore+ biopol)mers such as PC* should be used instead of traditional pol)mers+ such as pol)st)rene+
as biopol)mers brea, down in the environment and do not accumulate as debris.
Ch1 Production of materials/ Revision notes / p. 3
Syllabus reference !ctober 2""2 version#
2. Some
scientists
continue to
research the
e$traction of
materials from
biomass to
reduce our
dependence on
fossil fuels
Students learn to:
discuss the need for
alternative sources of the
compounds presently
obtained from the
petrochemical industry
explain what is meant by a
condensation polymer
describe the reaction involved
when a condensation polymer
is formed
describe the structure of
cellulose and identify it as an
example of a condensation
polymer found as a maor
component of biomass
identify that cellulose
contains the basic carbon-
chain structures needed to
build petrochemicals and
discuss its potential as a raw
material
Students:
use available evidence to
gather and present data from
secondary sources and
analyse progress in the
development and use of a
named biopolymer! "his
analysis should name the
specific en#yme$s% used or
organism used to synthesise
the material and an
evaluation of the use or
potential use of the polymer
produced related to its
properties
Comparison between polymerisation of ethylene and polymerisation of glucose
pol)merisation of eth)lene pol)merisation of glucose
5)pe of reaction
Pol)mer
?eatures monomer units chemicall) 6oin b)
7opening out8 their double bonds to form
single bonds between neighbouring units
* %%%%%%%%%%% molecule is released
when a monomer is added
&quation
Ch1 Production of materials/ Revision notes / p. .
=sing the date from the table+ what would be the appro'imate molecular weight of a pol)mer made from
11 glucose monomers !C
2
/
1
#
2
"D
4olecular mass of glucose - 1E1
4olecular mass of water - 1E
11 water molecules are released from the pol)merisation of 11 glucose monomer
4olecular mass nof pol)mer - 11!1E1" <11!1E" - 1E11
Ch1 Production of materials/ Revision notes / p. 2
Assess the suitability of biomass to reduce our dependence on fossil fuels
?ossil fuels such as crude oil are used to produce compounds which are used as source of
%%%%%%%%%%%%%%% and %%%%%%%%%%%%%%%%% for industr).
*s fossil fuels are %%%%%%%%%%%%%%%%%%%% resource+ the) could be completel) used up within a few
decades. *lternate energ) source has to be found to replace fossil fuels.
Biomass is a fuel source made up of %%%%%%%%%%%%%%%%%% and lignin from plants. 5he cellulose
components can be converted to glucose b) acid h)drol)sis.
@lucose can be then %%%%%%%%%%%%%%%%% to ethanol using )east.
&thanol can be used as a %%%%%%%%%%%%%%%on its own.
C
2
H
5
OH
(l)
+ 3O
2 (g)
2CO
2 (g)
+ 3H
2
O
(g)
+ energy
&thanol can also be deh)drated to form %%%%%%%%%%%%%% b) using %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
as catal)st. &th)lene can be used as a starting material to produce pol)eth)lene and other plastics.
5he problem with using biomass is that cellulose needs to be grown. 5his ma) result in a large amount of
land clearing. 5he acid h)drol)sis of cellulose is ver) inefficient and polluting and not currentl)
economicall) viable.
Ch1 Production of materials/ Revision notes / p. F
%. !ther resources,
such as ethanol, are
readily available
from renewable
resources such as
plants
Students learn to:
describe the dehydration
of ethanol to ethylene
and identify the need for
a catalyst in this process
and the catalyst used
describe the addition of
water to ethylene
resulting in the
production of ethanol and
identify the need for a
catalyst in this process
and the catalyst used
describe and account for
the many uses of ethanol
as a solvent for polar and
non-polar substances
outline the use of ethanol
as a fuel and explain why
it can be called a
renewable resource
describe conditions under
which fermentation of
sugars is promoted
summarise the chemistry
of the fermentation
process
define the molar heat of
combustion of a
compound and calculate
the value for ethanol
from first-hand data
assess the potential of
ethanol as an alternative
fuel and discuss the
advantages and
disadvantages of its use
identify the &'()C
nomenclature for
straight-chained alkanols
from C* to C+
Students:
process information from
secondary sources such
as molecular model kits,
digital technologies or
computer simulations to
model:
o the addition of
water to ethylene
o the dehydration of
ethanol
process information from
secondary sources to
summarise the processes
involved in the industrial
production of ethanol
from sugar cane
process information from
secondary sources to
summarise the use of
ethanol as an alternative
car fuel, evaluating the
success of current usage
solve problems, plan and
perform a first-hand
investigation to carry out
the fermentation of
glucose and monitor
mass changes
present information from
secondary sources by
writing a balanced
equation for the
fermentation of glucose
to ethanol
identify data sources,
choose resources and
perform a first-hand
investigation to
determine and compare
heats of combustion of at
least three liquid alkanols
per gram and per mole
Ch1 Production of materials/ Revision notes / p. E
Conversion between ethanol and ethylene
!1" deh)dration of ethanol to form eth)lene with concentrated sulfuric acid /phosphoric acid as
catal)st
Write an equation for the deh)dration of ethanol$ !indicate catal)st used"
!" h)dration of eth)lene to form ethanol with dilute sulfuric acid / phosphoric aid as catal)st
Write an equation for the h)dration of eth)lene$ !indicate catal)st used"
ermentation of sugar
Write an equation for the fermentation of glucose$
H = -ve
The conditions that promote the ermentation o s!gar are"
o #east to provide en$ymes
o %il!te a&!eo!s s!gar sol!tion
o Temperat!re in range o 3'
o
C to ('
o
C
o )naero*ic environment (a*sence o o+ygen)
Combustion of ethanol
,rite an e&!ation or the complete com*!stion o ethanol
-thanol is a _________________ reso!rce"
o -thanol can *e derived rom non-ossil !el so!rces. s!ch as the
ermentation o s!gar
o The s!gar can *e derived rom
s!gar cane
starch (mainly rom corn crops)
Ch1 Production of materials/ Revision notes / p. G
*acterial decomposition o cell!lose (c!rrently not commercially
via*le)
Ch1 Production of materials/ Revision notes / p. 11
!thanol is e"tensively used as a solvent
&thanol is widel) used as a solvent due to its structure. &thanol can act as a solvent for both %%%%%%%%%%%%%
and %%%%%%%%%%%%%%%%%%%%%%%% substances. &thanol is a small molecule. 5he ethanol molecule has a polar
%%%%%%%%%%%%%%%%%% end and a non9polar %%%%%%%%%%%%%%%%%% end.
5he polar end dissolves polar substances b) %%%%%%%%%%%%%%%% or %%%%%%%%%%%%%%%%%%%%%%% interactions.
5he non9polar end can dissolve non9polar substances b) %%%%%%%%%%%%%%%%%%%%%%% forces.
Comparison between water and ethanol as solvent
Water &thanol
Water is a small %%%%%%%%%%%%% molecule
containing onl) polar covalent bonds.
&thanol is also a small molecule but it contains
both %%%%%%%%%%%% and %%%%%%%%%%%%%%%%%%%
ends.
:t is attracted to other substances b) strong
h)drogen bonds and dipole9dipole attractions.
5his ma,es it an e'tremel) good solvent for
%%%%%%%%%%%%%%%%%%%%%%%% and some ionic
compounds. 7Ci,e dissolves %%%%%%%%%%%8.
5he polar end dissolves polar substances b) /<bonds
or dipole9dipole interactions. 5he non9polar end can
dissolve non9polar substances b) dispersion forces.
5hese properties ma,e ethanol an e'tremel) good
solvent for both polar and non9polar substances.
Ch1 Production of materials/ Revision notes / p. 11
#pecific heat and molar heat of combustion
$$$$$$$$$$$$$$$$$$$$$$$$$$$$ of a compound is the quantit) of heat required to increase the
temperature of 1g of the compound b) 1
o
C.
The ________________________________________ is the heat energy released /hen one
mole o a s!*stance !ndergoes complete com*!stion /ith o+ygen at a press!re o
0'013 23a (or 0 atmosphere). /ith the 4nal prod!cts *eing CO
2
and H
2
O
Ch1 Production of materials/ Revision notes / p. 1
!"periment to measure heat of combustion
Apparatus
Braw an e'perimental set which can be used to measure the molar heat of combustion of ethanol.
%esults:
4ass of water 011 g
:nitial mass of burner 100.1 g
?inal mass of burner 10.1. g
:nitial temperature of water ..1
o
C
?inal temperature of water 3...
o
C
Calculation:
q - mc H5 >pecific heat capcacit) of water - 3.1E '11
0
I ,g
91
J
91
=se the data above the find the molare heat of combustion of ethanol.
!"perimental errors
9 /eat loss to environment and heat lost in heating up the apparatus rather than water
9 :mpurities in the fuel
9 :ncomplete combustion of alcohol
5herefore+ e'perimental heat of combustion is significantl) %%%%%%%%%%%%% than the published value.
&ays to limit heat loss from apparatus
9 :nsulate bea,er of water
9 =ses an appropriate lid covering for bea,er
9 &nsure a minimal appropriate distance between spirit burner+ flame and bea,er
Ch1 Production of materials/ Revision notes / p. 10
Ethanol is carbon neutral fuel while fossil fuel is not. Discuss.
-thanol is prod!ced *y ermentation o s!gary crops s!ch as s!gar cane and corn1
C
5
H
02
O
5
(a&) 2C
2
H
5
OH(l) + 2CO
2
(g)
-thanol /hen com*!sted. orm car*on dio+ide and /ater and release heat and is
th!s a !se!l !el1
C
2
H
5
OH(l) + 3O
2
2CO
2
+ 3H
2
O
To orm s!gars in the 4rst place. these crops !ndergo photosynthesis /here car*on
dio+ide is a*sor*ed1
5CO
2
(g) + 5H
2
O(l) C
5
H
02
O
5
(s) + 5O
2
(g)
Thereore theoretically. the CO2 released /hen ethanol is com*!sted is *alanced *y
that a*sor*ed d!ring photosynthesis1
CO2 is tho!ght to *e the main ca!se o glo*al /arming and thereore ethanol
considered to *e car*on ne!tral in that it does not lead to net increase in car*on
dio+ide in the atmosphere1
The com*!stion o ossil !els s!ch as octane does lead to an increase in CO2
*eca!se the photosynthesis that prod!ced the s!gars that t!rned into ossil !els
occ!rred millions o years ago1
Ho/ever. ossil !els are !sed in the arming process and in the distillation and
distri*!tion o ethanol1
Th!s. ethanol as a !el does lead to a net increase in car*on dio+ide levels1
Ch1 Production of materials/ Revision notes / p. 13
&. !$idation'
reduction
reactions are
increasingly
important as a
source of
energy
Students learn to:
explain the displacement of
metals from solution in terms
of transfer of electrons
identify the relationship
between displacement of
metal ions in solution by
other metals to the relative
activity of metals
account for changes in the
oxidation state of species in
terms of their loss or gain of
electrons
describe and explain galvanic
cells in terms of
oxidation,reduction reactions
outline the construction of
galvanic cells and trace the
direction of electron flow
define the terms anode,
cathode, electrode and
electrolyte to describe
galvanic cells
Students:
perform a first-hand
investigation to identify the
conditions under which a
galvanic cell is produced
perform a first-hand
investigation and gather first-
hand information to measure
the difference in potential of
different combinations of
metals in an electrolyte
solution
gather and present
information on the structure
and chemistry of a dry cell or
lead-acid cell and evaluate it
in comparison to one of the
following:
o button cell
o fuel cell
o vanadium redox cell
o lithium cell
o liquid unction
photovoltaic device $eg
the -rat#el cell%
in terms of:
o chemistry
o cost and practicality
o impact on society
o environmental impact
solve problems and analyse
information to calculate the
potential .
/
requirement of
named electrochemical
processes using tables of
standard potentials and half
equations
Ch1 Production of materials/ Revision notes / p. 1.
Redo' reaction !o'idation9reduction reaction"
Oxidation = _______________ of electrons. (OIL)
Reduction = _________________ of electrons (RIG)
The o+idation and red!ction have to occ!r together1
)n oxidising agent 6666666666666666 electrons rom another reactant in a
redo+ reaction1 7t ca!ses o+idation o another reactant and itsel is
66666666666666666661
) reducing agent 6666666666666666666666 electrons to another reactant in a
redo+ reaction1 7t ca!ses the red!ction o another reactant and itsel is
6666666666666666661
Dislace!ent reaction (an exa!le of redox reaction)"
)n e+periment /as perormed to
investigate the relative activity o
metals1 The *ea2er initially contained
25'1' m8 o '1'5' mol 8
1
copper s!late
sol!tion1
Exected results"
Changes in the colo!r o the sol!tion"
Changes on the piece o $inc metal"
Exlanations"
9inc is a more 666666666666666 metal than copper1 9inc /ill 6666666666666666666 the
copper ions in coper s!late sol!tion 1 The more active metal has a greater tendency
to *e 666666666666666666661 9inc metal 66666666666666666666666 electrons to orm $inc
ions /hich goes into the sol!tion1 Th!s $inc is 6666666666666666661
;n
!s"
;n
+
!aq"
+ e
9
!o'idation"
Copper ions /ill 6666666666666666666666 the electrons released *y $inc to orm
666666666666 66666666666 /hich is the red-*ro/n deposit1 Thereore copper ions are
666666666666666661
Cu
+
!aq"
+ e
9
Cu
!s"
!reduction"
Write an overall reaction
!Redo'"
The copper (77) ions are *l!e1 )s they are converted into solid copper. C!2+ ions
concentration is 666666666666666666661 This e+plain /hy colo!r o the sol!tion
*ecomes lighter1
Ch1 Production of materials/ Revision notes / p. 12
Ch1 Production of materials/ Revision notes / p. 1F
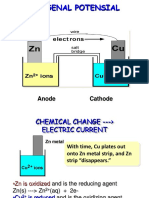
A galvanic cell
) gal#anic cell (:oltaic;%aniel) is an electrochemical cell that
66666666666666666666 electrical energy rom o+idation ; red!ction (redo+)
reactions ta2ing place /ithin the cell1
7t consist o t/o hal 6666666666666. the o+idation hal cell and red!ction hal
cell1
) hal cell consists o an 66666666666666666 and an 6666666666666666666661
The electrolyte sol!tions in the hal cells are <oined together
o *y a poro!s *arrier or
o 6666666666666666666666666 (a paper soa2ed /ith sat!rated =>O
3
sol!tion)
/hich allo/s the migration o ions to complete the circ!it1
o The t/o hal-cells may !se the same electrolyte. or they may !se di?erent
electrolytes1
The electrodes are <oined together *y an 6666666666666666666666666 /hich
allo/s the @o/ o 66666666666666666666 to complete the electrical circ!it1
-lectrons @o/ rom 666666666666666 to 66666666666661
o )ctive electrodes are made o the corresponding metal o the salt sol!tion1
o eg1 ) C!
2+
electrolyte !ses a C! metal electrode1
o 3assive electrodes do not ta2e part in the reaction
o are made o 6666666666666666666666 or 666666666666661
One electrode is the anode. the other is cathode1
o (a))node"
o O+idation occ!rs at anode
o )node is negative
o )node disintegrates
)> OA
o (*)Cathode"
o Bed!ction occ!rs at cathode
o Cathode is positive
o Colid deposits on cathode
B-% C)T
Ctandard conditions in a galvanic cell"
o The electrolytes concentrations are 0 D
o the temperat!re is 25EC and
o the press!re is 0 atmosphere (=0'013 23a)1
Ch1 Production of materials/ Revision notes / p. 1E
Ch1 Production of materials/ Revision notes / p. 1G
Ch1 Production of materials/ Revision notes / p. 1
Comparison between a dry cell and a button cell
* dr) cell * button cell
%ry cell F!tton Cell
cost and
practicality
:ery cheap ((' cents)
Beadily availa*le (p!rchased rom
s!permar2ets)1
Gsed in torches. T: remotes. porta*le
C% players. alarm cloc2s. etc1
Ho/ever they have a short lie1
They cannot *e recharged1
They are *ig. *!l2y and heavy
compared to the *!tton cell. ho/ever
&!ite small and light compared to the
lead-acid *attery1
o 0ery small and light
o 1elatively long life
o Silver is an expensive
metal, making the cell slightly
more expensive than the dry
cell, however it is still
relatively cheap, with a rough
price of about 23-24!
o Constant output voltage
o 5on-rechargeable
impact on
society
)s they are relatively small and light.
they can are !sed to ma2e porta*le
appliances1 Hor e+ample. a porta*le
radio. can impact society as it can *e
!sed to inorm people on *oats o
approaching storms1 This /arning can
save them rom the storm1
o Small si#e has allowed for
miniature electric appliances
o 5on-toxic nature has
allowed for use inside the body
o .!g! Their !se in
pacema2ers has allo/ed
people to live longer1
environment
al impact
The dry cells are non-rechargea*le and
as they donIt last long. many end !p in
land4ll1 The o!ter $inc case corrodes
and $inc ions escape into the
s!rro!nding soil1 ) high concentration o
$inc ions is to+ic to vegetation1
o Have to be
discarded,recycled after one
use
o (otassium hydroxide
electrolyte is caustic
o 5o highly toxic materials
that will harm the
environment
Ch1 Production of materials/ Revision notes / p. 1
(. )uclear
chemistry
provides a
range of
materials
Students learn to:
distinguish between stable
and radioactive isotopes and
describe the conditions under
which a nucleus is unstable
describe how transuranic
elements are produced
describe how commercial
radioisotopes are produced
identify instruments and
processes that can be used to
detect radiation
identify one use of a named
radioisotope:
o in industry
o in medicine
describe the way in which the
above named radioisotopes
are used and explain their
use in terms of their
properties
Students:
process information from
secondary sources to describe
recent discoveries of
elements
use available evidence to
analyse benefits and
problems associated with the
use of radioactive isotopes in
identified industries and
medicine
Ch1 Production of materials/ Revision notes / p.
'sotopes
Isotoes are atoms o the same element /ith di?erent n!m*ers o
6666666666666666666661
They have the same 666666666666666666 n!m*er *!t di?erent
666666666666666 n!m*er1
)s they have same electron con4g!rations. they have the same
666666666666666 properties1
Di$erence between stable and radioacti#e isotoes
) sta*le isotope is one that doesnIt disintegrate1
) radioactive isotope is an !nsta*le isotope that
___________________________________________________________ with
a change in the ______________________________________________1
Conditions under which a nucleus is unstable
1. atom with a %%%%%%%%%%%%%%%%%%%%%%%%%%%%% !atomic number K %%%%%%%%%%%%"
. high %%%%%%%%%%%%%%%%%%%%%%%%% ratio
Alpha and beta decay
=nstable nuclei !radioisotopes" undergo radioactive deca) to form more stable daughter nuclei.
!1" *lpha deca)
:t involves emitting a %%%%%%%%%%%%%%%%%% nucleus from the isotope nucleus
!" Beta deca)
:t involves the conversion of a neutron into a %%%%%%%%%%%%%%%% and an %%%%%%%%%%%%
(3) Jamma radiation
Jamma radiation accompanies most other types o radioactive decay to
red!ce the 666666666666 o the n!cle!s1
Ch1 Production of materials/ Revision notes / p. 0
Transuranic elements
%ransuranic elements are elements /ith atomic n!m*ers greater than
66666666666666666K that is 9 L 66666666 (more than 666666666 protons)1
>one o these elements are sta*le and each decays radioactively into other
666666666666666661
Production of transuranic elements
)ll trans!ranic elements are arti4cially prod!ced *y"
(0) _____________________________ in a n!clear reactor"
>!clear reactors prod!ce lots o ne!trons rom 4ssion reactions1
o Target n!cle!s is *om*arded /ith ne!trons in a n!clear reactor1 The
target n!cle!s a*sor*s the ne!trons to orm a radioisotope1
o E.g. for!ation of &etuniu!'()* fro! uraniu!'()+"
,hen Grani!m-23M is *om*arded /ith ne!trons in a n!clear
reactor. it can *e converted to G-23N1
Grani!m-23N can then convert to nept!rni!m-23N *y *eta decay.
in /hich one ne!tron converts to a proton. an electron (*eta
particle)
>ept!rni!m-23N can then convert to pl!toni!m-23N *y *eta
decay. in /hich one ne!tron converts to a proton. an electron
(*eta particle)
o
(2) _________________________________ in article accelerators"
o 5here are three t)pes of accelerators$ %%%%%%%%%%%%%%%%%%%%%%%+ %%%%%%%%%%%%%%%% and
s)nchrotrons.
o &ach uses alternating %%%%%%%%%%%%%%%%%% and %%%%%%%%%%%%%%%%%%%%% fields to accelerate
%%%%%%%%%%%%%%%%% particles !such as protons+ alpha particles or nuclei of larger atoms" at
high speed to penetrate a target nucleus to produce a %%%%%%%%%%%%%%%%%%9deficient
radioisotope
o &.g. production of prod!ction o %armstadti!m
Ch1 Production of materials/ Revision notes / p. 3
Application of radioisotopes in industry and medicine in relation to their properties:
Radioisotopes have made man) procedures easier+ safer+ more cost effective+ more accurate and more
reliable for societ). /owever there are ris,s and problems associated with their use.
()* +edical use of radioisotopes
e.g. :odine9101
9 to treat or diagnose th)roid disorders
Radioisotopes usuall) have the same chemical properties to non9radioactive isotopes. 5hus when iodine9
101 is ingested+ the) will concentrated in th)roid gland.
:odine9101 has a half9life of 6ust over E da)s and emit L9particles and M9ra)s as it deca)s. 5he half9life is
long enough to be transported from where it is produced to where it is to be used+ while short enough to
minimise a patient8s e'posure to radiation.
5he L9particles can penetrate and destro) abnormal tissues+ while the M9ra)s allow e'ternal imaging to
ensure the target region has been reached.
(,* 'ndustrial use of radioisotopes
>odium93 has a ver) short half9life and so it disappears rapidl) after its use and is not a threat to the
environment. 5hus it can be used to detect lea,s in underground water or gas pipes.
Cobalt921 releases M9ra)s which is effective in destro)ing biological molecules such as bacteria. >o it can
be used to sterilise food and medical supplies+ such as dressing and bandages.
*mericium931 is used in smo,e detectors. *mericium931 emits alpha particles. :t has a long half9life of
30 )ears meaning that it never needs replacing. 5he alpha particles do not themselves pose a health
haNard as the) are absorbed in a few centimetres of air or b) the structure of the detector.
%isks of using radioisotopes:
Radioisotopes present problems if mishandled or not shielded properl) as radiation can cause tissue
damage+ cancers and or genetic mutation. People must therefore ta,e care to monitor and minimise their
e'posure to radioisotopes.
#ther possible problems include accidents during the production of radioisotopes at nuclear reactors and
disposal of radioactive wastes.
/owever if care is ta,en and radioisotopes are used properl)+ and the problems are managed adequatel)+
the benefit of radioisotopes outweigh the problems.
Ch1 Production of materials/ Revision notes / p. .
*n unstable isotope can undergo %%%%%%%%%%%%%%%%%%%%% or %%%%%%%%%%%%%%%%%%%%% in order to
achieve greater %%%%%%%%%%%%%%%%%%%%%%.
&'ample of alpha deca)$
&'ample of beta deca)$
Ch1 Production of materials/ Revision notes / p. 2
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Ch4 PercentageDocument7 pagesCh4 PercentageWing ChuNo ratings yet
- Chemistry 1987 Paper 1Document13 pagesChemistry 1987 Paper 1Wing ChuNo ratings yet
- Roundabouts: Roundabout Traffic SignsDocument2 pagesRoundabouts: Roundabout Traffic SignsWing ChuNo ratings yet
- Unfair Contract Terms GuideDocument28 pagesUnfair Contract Terms GuideWing ChuNo ratings yet
- Production of Materials Revision 2 Chapter 1Document12 pagesProduction of Materials Revision 2 Chapter 1Wing ChuNo ratings yet
- How To Build A DeckDocument4 pagesHow To Build A DeckWing ChuNo ratings yet
- NSW Housing Code ChecklistDocument12 pagesNSW Housing Code ChecklistWing ChuNo ratings yet
- A Guide To Australian Government Payments 2014Document44 pagesA Guide To Australian Government Payments 2014Wing ChuNo ratings yet
- New Tenant ChecklistDocument2 pagesNew Tenant ChecklistWing ChuNo ratings yet
- JNTO Japanese Hot SpringsDocument5 pagesJNTO Japanese Hot Springswerawatb819No ratings yet
- Winter OlympicsDocument10 pagesWinter OlympicsWing ChuNo ratings yet
- Skiing in JapanDocument6 pagesSkiing in JapanWing ChuNo ratings yet
- Redox WsDocument5 pagesRedox WsWing ChuNo ratings yet
- 2006 Mathematics Paper1Document12 pages2006 Mathematics Paper1Wing ChuNo ratings yet
- 2001 Cranbrook Trial SolutionsDocument6 pages2001 Cranbrook Trial SolutionsWing ChuNo ratings yet
- Redox HandoutDocument1 pageRedox HandoutWing ChuNo ratings yet
- 2001 Cranbrook TrialDocument7 pages2001 Cranbrook TrialWing ChuNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Engineering Corrosion OH-4: University of Hafr Al BatinDocument41 pagesEngineering Corrosion OH-4: University of Hafr Al BatinHussain Al-DawoodNo ratings yet
- Electrochemistry PDFDocument55 pagesElectrochemistry PDFVishal NaikNo ratings yet
- Electrochemistry Galvanic Cell NewDocument48 pagesElectrochemistry Galvanic Cell Newralphkirby.felicitaNo ratings yet
- Physical Sciences P2 May-June 2017 EngDocument19 pagesPhysical Sciences P2 May-June 2017 EngThando ChebaseNo ratings yet
- 8062Document27 pages8062Aashish Moyal100% (1)
- Siti Nur FatihahDocument6 pagesSiti Nur Fatihahtean_ego88% (8)
- Chemistry NotesDocument82 pagesChemistry Notesanuteck1No ratings yet
- History of Electrochemistry Electrolysis Cells/galvanic Cells Electrode/Electrochemical Process Anode/Cathode Anions/CationsDocument8 pagesHistory of Electrochemistry Electrolysis Cells/galvanic Cells Electrode/Electrochemical Process Anode/Cathode Anions/Cationslapenoenriquez1No ratings yet
- Lab Report Electrochemical CellsDocument6 pagesLab Report Electrochemical CellsemiNo ratings yet
- Low Volatge and BatteryDocument74 pagesLow Volatge and BatteryOladokun Sulaiman OlanrewajuNo ratings yet
- 86 95Document167 pages86 95Ashwin KirtaneNo ratings yet
- Chemistry Assignment.......Document11 pagesChemistry Assignment.......Masood MughalNo ratings yet
- 03.thermodynamics in Corrosion EngineeringDocument33 pages03.thermodynamics in Corrosion EngineeringAsad AlfautoreNo ratings yet
- Electrochemistry NotesDocument54 pagesElectrochemistry NotesAkash Roy67% (6)
- Lab Manual Ebt 251Document29 pagesLab Manual Ebt 251Ahmad Helmi AdnanNo ratings yet
- Chem131 SyllabusDocument7 pagesChem131 SyllabusCelape CabanesNo ratings yet
- CH-110, Lecture 1Document29 pagesCH-110, Lecture 1Naveed TanoliNo ratings yet
- Chemistry Study Material - 2011-12Document246 pagesChemistry Study Material - 2011-12Harsh BhambhaniNo ratings yet
- Hello ChemDocument14 pagesHello ChemAndreiFoxNo ratings yet
- Galvanic Cells IntroDocument3 pagesGalvanic Cells IntroSana SyedNo ratings yet
- Thermodynamics of CorrosionDocument59 pagesThermodynamics of Corrosionrajkumar baskeyNo ratings yet
- General Chemistry IV 177 PtsDocument17 pagesGeneral Chemistry IV 177 PtsXyleen GregolaNo ratings yet
- Redox Reactions Practice WorksheetDocument7 pagesRedox Reactions Practice WorksheetPeter Missole100% (1)
- Class 12 Chemistry: Common mistakes in electrochemistry examDocument45 pagesClass 12 Chemistry: Common mistakes in electrochemistry examshilswapanNo ratings yet
- Physics Action Verbs Definitions and ExamplesDocument100 pagesPhysics Action Verbs Definitions and ExamplesWave NewNo ratings yet
- C12SB764Document2 pagesC12SB764Tish BarnesNo ratings yet
- Chemistry Grade 12Document180 pagesChemistry Grade 12Nam Hua100% (1)
- Chem PDFDocument96 pagesChem PDFNikhilNo ratings yet
- POTENSIAL KorosiDocument33 pagesPOTENSIAL KorosiLisa AndrianiNo ratings yet
- Electromotive force explainedDocument12 pagesElectromotive force explainedLani_Reyes_7449No ratings yet