Professional Documents
Culture Documents
The Bimolecular Binding Event
Uploaded by
studycamOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Bimolecular Binding Event
Uploaded by
studycamCopyright:
Available Formats
The bimolecular binding event
determination of the binding constant
experimental conditions
data fitting
binding stoichiometry
analytical techniques: scope and limitations
practical considerations
H + G H G
H S (H G) S G S
+
in reality
K
[HG]
[H][G]
K
a
=
[HG]
[H][G]
K
d
= (M
-1
) (M)
S S
+
explicit solvent is not used because G for the association constant
reflects the stability of solvated H and G relative to solvated HG and released solvent
G
= -RTln(K
a
)
assuming activity = concentra\ tion
the ratios of H, G, and HG depend on the initial concentrations of H
0
and G
0
K
a
= 100 M
-1
H
0
(M) G
0
(M) H (M) G (M) HG (M) /%
1x10
-3
1x10
-3
0.9x10
-3
0.9x10
-3
0.1x10
-3
/10
1x10
-2
1x10
-2
6.6x10
-3
6.6x10
-3
4.4x10
-3
/44
1x10
-1
1x10
-1
27x10
-3
27x10
-3
73x10
-3
/73
1 1 95x10
-3
95x10
-3
905x10
-3
/91
course
0,1 0,3 0,5 0,7 0,9
Total concentrationof A
0
20
40
60
80
100
%
f
o
r
m
a
t
i
o
n
r
e
l
a
t
i
v
e
t
o
A
[H] and [G]
[HG]
K
d
(1/K
a
) is a convenient reference point for estimating the amount of complex
K
a
= 100 M
-1
K
d
= 1x10
-2
M = 10 mM
course
0,1 0,3 0,5 0,7 0,9
Total concentration of A
0
20
40
60
80
100
%
f
o
r
m
a
t
i
o
n
r
e
l
a
t
i
v
e
t
o
A
[H]
0
= [G]
0
= 20 mM [H] = [G] = 10 mM and [HG] = 10 mM
At K
d
, [H] = [G] = [HG] = K
d
(!! only in case [H]
0
= [G]
0
!!)
G
= -RTln(K
a
)
K
a
(M
-1
) G
0
(kJ.mol
-1
)
1 0
10 -5.8
100 -11.5
1000 -17.3
10000 -23.0
100000 -28.8
at T = 301 K (R = 8.314 J.mol
-1
.K
-1
)
0
5
10
15
20
25
30
35
0 1 2 3 4 5 6
-
(
k
J
m
o
l
-
1
)
log K
a
H + G H G
K
a
how to determine the concentration of each species ?
if H, G, and HG are all known, then K is easily calculated
[HG]
[H][G]
K
a
=
regrettable this is hardly ever the case
determination of the binding constant K
a
almost all experimental methods to measure binding constants rely on the analysis
of a binding isotherm.
A binding isotherm is the theoretical change in the concentration of one component
as a function of the concentration of another component at constant temperature.
The concentrations are experimentally determined (e.g. NMR, UV/vis, etc) and fitted to
the theoretical binding isotherm.
0,1
0 0,2 0,4 0,6 0,8 1,0
Total concentration of A
0
20
40
60
80
100
%
f
o
r
m
a
t
i
o
n
r
e
l
a
t
i
v
e
t
o
B
K
a
= 100 M
-1
[H]
0
= 0.01M
example
Titration experiments
Typically a titration is performed holding the concentration of one species (H) constant
while varying the concentration of the other (G).
During the course of this titration, the physical changes in the system are monitored,
usually spectroscopically, and this change is then plotted as a function of guest added
to host (equivalent guests).
The mathematical model used to obtain the association constant is usually developed
from realising that the physical change (Y, e.g., a NMR shift or a change in UV-Vis
absorbance) observed is correlated to the concentration of the complex [HG] as Y =
f[HG], or in some cases, the free host f[H] or the free guest f[G].
The physical change (Y) being monitored can usually be described as the aggregate of the
individual components according to eqn (7) as a function of concentration (e.g., for UV-Vis
spectroscopy) or eqn (8) as a function of mole fractions f
X
(f
X
defined as: f
X
= [X]/[X]
0
) in
the special case of NMR.
Y = Y
H
[H] + Y
G
[G] + Y
HG
[HG]
Y = Y
H
f
H
+ Y
G
f
G
+ Y
HG
f
HG
[H] = [H]
0
[HG]
[G] = [G]
0
[HG]
[HG]
([H]
0
[HG])([G]
0
[HG])
K
a
=
[HG]
[H][G]
K
a
=
Example
only HG has a particular absorption band in the UV/vis spectrum.
Thus, A =
HG
[HG]
[HG]
([H]
0
[HG])([G]
0
[HG])
K
a
=
[HG]
[H]
0
[G]
0
[HG][G]
0
- [HG][H]
0
+[HG]
2
K
a
=
K
a
([H]
0
[G]
0
[HG][G]
0
- [HG][H]
0
+[HG]
2
) = [HG]
K
a
[HG]
2
(K
a
[G]
0
-K
a
[H]
0
+1)[HG] +K
a
[H]
0
[G]
0
= 0
thus
this expresses [HG] as a function of K
a
, which is the only unknown !!
Example, only HG has a particular absorption band in the UV/vis spectrum.
Since, A =
HG
[HG]
and
Then A = f (
HG
, K
a
)
The power of this equation should not be understated as we can now start to develop
solutions that require only the knowledge of the total (or initial) concentrations of the
host and guest ([H]
0
and [G]
0
) in addition to the association constant (K
a
) and the physical
properties (Y) that are changing (Y) during the course of the titration.
Y = Y
H
f
H
+ Y
G
f
G
+ Y
HG
f
HG
If we further assume that one of the components is silent e.g., a non-absorbing free
guest [G], we can simplify the equation to
which, since f
HG
= [HG]/[H]
0
and f
H
= 1- f
HG
, can be further simplified to
Y=Y
H
+([HG]/[H]
0
)(Y
HG
- Y
H
)
and, finally
Y = Y
H
f
H
+ Y
HG
f
HG
in which Y = Y-Y
H
and Y
HG
= Y
HG
-Y
H
(Old-fashioned) Shortcuts to the binding constant
Older references and textbooks are full of examples on how some of the above
expressions and equations can be simplified or transformed to linear equations
(y = a + bx) which could then be plotted by hand to obtain the K
a
and other parameters
of interest by inspection of the slope and intercepts.
BenesiHildebrand plot
(determination of binding constants based on absorbance)
assuming that [G]
0
>> [H]
0
(and thus A
H
<< A
G
)
with this gives (Lambert-Beer)
considering that [G]
0
>> [H]
0
one can assume that [G]=[G]
0
and this
with =
HG
-
G
[H] = [H]
0
[HG]
[HG]
[H][G]
K
a
=
[HG]
[G]([H]
0
-[HG])
K
a
= and
K
a
([G]([H]
0
-[HG]))-[HG]=0
which is
K
a
[G][H]
0
-K
a
[G][HG]-[HG]=0
or
The binding isotherm can be rewritten as
Together this gives
and finally
(assuming that [G] = [G]
0
)
intercept
slope
1
b[H]
0
K
a
Other examples include Lineweaver-Burke plots, Scatchard plots, etc.
There are two key problems associated with using these linear transformations that
make their use highly questionable:
(i) they violate some of the fundamental assumption of linear regression by
distorting the experimental error
(ii) they frequently involve assumptions and shortcuts (such as assuming that [G]
0
>> [H]
0
or that Y
HG
= Y at the end of titration (i.e., the complex is fully formed at
the end of titration - which would then help to give Y
HG
). These assumptions
are often not valid and distort the results.
The non-linear regression approach with exact solutions of the quadratic equation
(see before) produces the most accurate results. This approach is not difficult with
modern computer technology and there is no real excuse for using old-fashion linear
transformations anymore!
Limitations
Scientist
0
0.0002
0.0004
0.0006
0.0008
0.001
0.0012
0.00E+00 5.00E-03 1.00E-02 1.50E-02 2.00E-02
Example: NMR titration
[G
0
]
o
b
s
(
p
p
m
)
6.8
7
7.2
7.4
7.6
7.8
8
8.2
0 0.005 0.01 0.015 0.02
Serie1
Serie2
K 1938.702 78
DH 7.00624 0.004
DHG 8.000867 6.00E-03
M
-1
ppm
ppm
[G
0
]
o
b
s
(
p
p
m
)
Experimental conditions
When a supramolecular titration study is carried out one has to first make a decision
on what technique is going to be used to follow the physical changes (Y) in the
system during the course of experiment. The two key concerns here should be:
i. The expected association constant(s).
ii. The expected physical changes (Y) upon association.
The expected association constant determines what concentration should be chosen for the
host system which in turn will have an influence on the choice of technique.
Wilcox, using a parameter defined as probability of binding (p), showed that it is vital to
collect as many data point as possible within the range: 0.2 < p < 0.8
with p defined according to
[HG]
([H]
0
[HG])([G]
0
[HG])
K
a
=
gives
K
a
=
p
[G]
0
([H]
0
+ [G]
0
)p+[H]
0
p
2
p
for [H]
0
=[G]
0
=0.001 M
near p =0 and p = 1, small errors in p (experimental error in determination of the
concentrations !!= results in large variations in K
a.
The best results are obtained in the region 0.2 < p < 0.8
EXAMPLE
p
for [H]
0
=0.001 M
K
a
= 2000 M
-1
0.00
0.20
0.40
0.60
0.80
1.00
1.00E-06 1.00E-05 1.00E-04 1.00E-03 1.00E-02 1.00E-01 1.00E+00
log [G]
0
0.00
0.20
0.40
0.60
0.80
1.00
0.00E+00 2.00E-03 4.00E-03 6.00E-03 8.00E-03 1.00E-02
[G]
0
for [H]
0
=0.001 M
K
a
= 2000 M
-1
p
Using
it is possible to calculate p for a range of [H]
0
, [G]
0
and K
a
values.
When the results are plotted for a fixed [H]
0
concentration (here 10
-5
M) as a
function of K
a
and [G]
0
/[H]
0
(equivalents of guest added) typically employed in
UV-Vis spectroscopy studies a revealing pattern appears with the shaded areas
indicating p in the range of 0.20.8 (note that this applies only to 1 : 1 binding
systems).
if K
d
> [H]
0
(hence K
a
fairly low) then a relatively large excess of [G]
0
is required to obtain
good p-values. In this situation it would be advisable to collect several data points in the
range of 150 equivalents of G added.
If K
d
> [H]
0
(hence K
a
fairly high) the only data points with good p-values are within the
range of [G]
0
o [H]
0
. In other words, it is essential to obtain as many points as possible
between 01 equivalents of G added.
If K
d
[H]
0
, good p-values are obtained almost anywhere within the range of 0 to >10
equivalent of G added. Note that when K
d
= [H]
0
= [G]
0
, then p = 0.38.
The fourth scenario to consider is when K
d
<<[H]
0
, i.e., by a factor of at least 100. Here, it
is not enough to look just at the p values obtained. Consider instead what is happening
with the non-linear portion of the resulting binding isotherms
Binding isotherms for different [H]
0
/K
d
ratios from 110 000.
The inset shows the region around 0.91.1 equivalents
added for [H]
0
/K
d
= 100010 000 only.
Here it becomes clear that once [H]
0
/K
d
> 100,
the nonlinear portion of the resulting binding
isotherms is restricted to a small region around
1 equivalent of guest added. With
[H]
0
/K
d
>1000 , it is clear that there is very little
information content in the isotherms.
When binding occurs under saturation
conditions, this implies that the experimental
conditions are NOT adequate for
determination of K.
NMR concentrations
UV-Vis concentrations
fluorescence concentrations
The suitable analytical technique for
determination of K
a
depends on its
value.
limit: [H]
0
/K
d
> 100
analytical techniques: scope and limitations
The most informative technique in most situations is
1
H NMR. Other forms (
13
C,
19
F etc.)
of NMR are also applicable. Apart from the quantitative information that an
NMR titration can yield, the relative shifts and changes in symmetry can often give
valuable information about how the host and guest(s) are interacting and the
stoichiometry of interaction. This information can be of significant benefit even
in situations where complete quantitative data cannot be obtained from the NMR
titration.
Classical approaches for data analysis of NMR titrations assume that the resonance () of
interest is the weighted average of the free host (H) and the bound host in the complex
(HG) in the experiment for a simple 1 : 1 system
obs
=
H
H
+
HG
HG
since
H
= 1 -
HG
obs
-
H
=
HG
(
HG
HG
)
NMR spectroscopy
since
this gives
NMR spectroscopy
With modern NMR instruments it is possible to obtain good quality spectra with sub-
millimolar concentrations (routinely now as low as 10
-4
M), suggesting that NMR is
suitable for K
a
up to and even above 10
6
M
-1
. Many literature references will state
that 10
5
M
-1
is the limit for NMR titration experiments.
With NMR, one has also to take into account the relative exchange rates within the
hostguest the relationship between the equilibrium association constant and the
kinetics on/off rates (K
a
=k
1
/k
-1
) and the timescale of the NMR experiment. The real
limiting factor for NMR titrations is therefore whether the system of interest is in the
fast or slow exchange region under the conditions used.
It may be tempting to think that in the (very) slow exchange region of NMR, one could
obtain an association constant directly from the relative ratios of the free and bound
host, however, can be difficult in practice due to complications that arise in the
intermediate-to-slow region with the size (amplitude) of the observed resonances and
the usual limitation of obtaining accurate (quantitative) integration from NMR
experiments.
UV-Vis spectroscopy
The second most common method for supramolecular titration experiment is probably
UV-Vis spectroscopy. With the right chromophore, host concentration in the sub-
micromolar (10
-7
M) can be applied, making the determination of association constants
as high as 10
9
M
-1
in simple 1 : 1 systems possible (albeit difficult) with K
d
/[H]
0
= 100 as
discussed above.
advantages
rapid. absorption and related phenomenon (fluorescence) occur on ps time scale or
faster in all cases faster than complex dissociation rate slow exchange
straightforward to correlate signal intensity to concentration (linear regime;
Lambert-Beer)
sensitive
disadvantage
titration by UV-Vis spectroscopy is particularly vulnerable to dilution and temperature
effects (all supramolecular titration experiments need some temperature control) and
the presence of impurities in either host or guest solutions.
requires chromophoric hosts or guests
fluorescence spectroscopy
The phenomenal sensitivity of this technique makes routine measurements in the sub-micromolar,
even nanomolar (nM) range possible and hence, fluorescence spectroscopy is ideal for the
determination of very large association constants (K
a
> 10
10
M
-1
).
Fluorescence is a particularly useful technique in the case when only one of the species in solution
is fluorescently active, i.e. when either the free host or guest is fluorescent silent or inactive and
the fluorescence of the remaining species is either turned off (quenched) or on upon
complexation.
If quenching plays a role, it is necessary to differentiate between static and dynamic (collisional)
quenching, with only the former of real significance for supramolecular binding studies.
Dynamic quenching is usually measured by plotting the ratio of the initial (F
0
) and measured (F)
fluorescence intensity ratio (F
0
/F) against the concentration of the quencher [Q] according to the
SternVolmer relation F
0
/F = 1 + K
SV
[Q], with K
SV
= the SternVolmer constant.
Unfortunately, pure 1 : 1 static quenching follows a nearly identical relation: F
0
/F = 1 + K
a
[Q], with [Q]
= the free concentration of the quencher (guest) and K
a
is the association constant of interest in
supramolecular binding studies.
In many cases the observed quenching is a mixture of both static and dynamic quenching which can
lead to some complication in the analysis of the titration data.
Swager et al. J. Am. Chem. Soc., 1995, 7017-7018
control
Stern-Volmer plots (F0/F=1+K[PQ
2+
] )
Small molecule sensing
2
Mean molecular weight (PDI)
6: 31 100 (1.6)
7: 65 400 (1.6)
8: 122 500 (1.8)
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
- - - 2
0
250
500
750
1000
0.0 1.0 2.0 3.0 4.0 5.0
F
I
(
a
.
u
.
)
[ATP
F
] (M)
ATP
F
(
ex
= 305 nm,
em
= 370 nm)
2.5 M
~ 18
[TACN Zn(II)] = 10 M
pH = 7.0, T = 25 C.
[TACN Zn(II)] = 10 M
pH = 7.0, T = 25 C.
G
= -RTln(K
a
)
From K to G and H and S (Van t Hoff analysis)
G
= H- T S
ln(K
a
) = -H/RT+ S/R
slope = -H/R
intercept = + S/R
problem: often small temperature interval possible + inversed temperature
Isothermal calorimetry (ITC)
Q = VH[HG]
or
dq = VH [HG]
fitting provides K and H
Determination of stoichiometry
The other aim of a supramolecular titration experiment is the determination of the
stoichiometry of the system.
Methods
(i) The method of continuous variations (Jobs method).
(ii) Consistency with the host structure and available information on the host
guest complex structure.
(iii) Specific experimental evidence such as isosbestic point(s).
(iv) Constancy of stability concentration as the concentration is varied, that is, the
success of a stoichiometric model to account for the data.
Jobs method
The idea behind it is simple; the concentration of a H
m
G
n
([H
m
G
n
]) complex is at
maximum when the [H]/[G] ratio is equal to m/n.
To do this, the mole fraction (f
G
) of the guest is varied while keeping the total
concentration of the host and guest constant ([H]
0
+[G]
0
=constant). The concentration of
the hostguest complex [H
m
G
n
] is then plotted against the mole fraction f
G
yielding a
curve with a maxima at f
G
= n/(m + n), which in the case of m = n (e.g., 1 : 1) appears at f
G
= 0.5
When there is more than one complex present, the Jobs method becomes
unreliable. This includes many situations with m/n = 1 : 2 or 2 : 1 as these usually
include two forms of complexes (e.g., HG and HG
2
) that have different physical
properties, hence the assumption that the physical property of interest (e.g.,
obs
)
is linearly dependent may not be valid. For similar reasons, the Jobs method is
likely to fail when either the host or guest aggregates in solution.
Limitations
This method is perhaps the simplest but often the most effective of all the approaches
available to determine the stoichiometry in hostguest complexes. In modern
supramolecular chemistry it is now rare not to have detailed information through X-ray
crystallography, 2D-NMR and Molecular Modelling about the structure of the host and
guest and, in some cases, even the hostguest complex itself. This structural information
can make the prediction of stoichiometry quite straightforward and accurate.
(ii) Consistency with the host structure and available information on the
hostguest complex structure.
This relies on specific evidence such as isosbestic points which can be used to confirm
that more than one type of complex is present and hence that simple 1 : 1 complexation
is not appropriate to describe the system if more than one isosbestic point is observed.
The converse is not necessarily true, i.e. the absence of more than one isosbestic point
cannot be used to rule out more complex stoichiometry such as 1 : 2 complex formation,
especially in cases where the cooperative (positive or negative) processes play a
significant role.
(iii) Specific experimental evidence such as isosbestic point(s).
(iv) Constancy of stability concentration as the concentration is varied, that is, the
success of a stoichiometric model to account for the data.
This method is probably the most generally applicable method for determining
stoichiometry.
Firstly, if anything other than 1 : 1 stoichiometry is suspected, the data should be fitted
to other plausible models (e.g., 1 : 2) and the quality of fit of the different models
compared in details, taking into account factors such as the increase in parameters in
the fitting process.
Secondly, and more importantly, it is strongly advisable to carry out the titration at
different concentrations and even with different techniques (e.g., NMR and UV-Vis). If a
particular model is successful at explaining the data at different concentrations then it
can be taken as very strong evidence for that model.
6.8
7
7.2
7.4
7.6
7.8
8
8.2
0 0.005 0.01 0.015 0.02
Serie1
Serie2
[G
0
]
o
b
s
(
p
p
m
)
1:1 model
K 1938.702 78
DH 7.00624 0.004
DHG 8.000867 6.00E-03
M
-1
ppm
ppm
6.8
7
7.2
7.4
7.6
7.8
8
8.2
0 0.005 0.01 0.015 0.02
Serie1
Serie2
1:2 model
[G
0
]
o
b
s
(
p
p
m
)
K 2615630 5.00E+05
DH 6.98578 0.02
DHG 8.169739 0.04
M
-1
ppm
ppm
[G
0
]
o
b
s
(
p
p
m
)
2:1 model
HHG
K 3566244 1.20E+06
DH 7.05578 0.02
DHG 7.92317 0.03
M
-1
ppm
ppm
6.8
7
7.2
7.4
7.6
7.8
8
8.2
0 0.005 0.01 0.015 0.02
S
S
HGG
dilution
0.00
0.20
0.40
0.60
0.80
1.00
1.20
0.00 5.00 10.00 15.00 20.00
H0=0.1 mM
H0=1 mM
H0=10 mM 1:1 model
HG
K 1938.702 78
DH 7.00624 0.004
DHG 8.000867 6.00E-03
M
-1
ppm
ppm
0.00
0.20
0.40
0.60
0.80
1.00
1.20
0.00 5.00 10.00 15.00 20.00
Serie1
Serie2
Serie3
K 3566244 1.20E+06
DH 7.05578 0.02
DHG 7.92317 0.03
M
-1
ppm
ppm
1:2 model
HGG
[G
0
]
o
b
s
(
p
p
m
)
[G
0
]
o
b
s
(
p
p
m
)
at lower concentrations
the difference between
the binary and ternary
complex become more
evident.
Setting up a titration experiment
weigh H stocksolution H
weigh G
(concentrated solution)
use to fill
stocksolution G
(contains H at the same concentration !)
1) measure initial spectrum
2) add aliquots of G
3) measure after each addition
You might also like
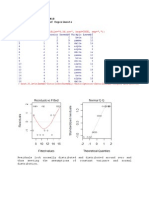
- Applied Linear Regression HomeworksDocument6 pagesApplied Linear Regression Homeworksstudycam100% (1)
- Experimental Design and Analysis Montgomerry HW#22Document4 pagesExperimental Design and Analysis Montgomerry HW#22studycam100% (1)
- ERROR ANALYSIS OF EXPERIMENTS A Manual For Engineering StudentsDocument49 pagesERROR ANALYSIS OF EXPERIMENTS A Manual For Engineering StudentsWaseem Ali AkbarNo ratings yet
- ExperimentosDocument680 pagesExperimentosGerardo Lopez100% (4)
- Aromatic InteractionsDocument32 pagesAromatic Interactionsstudycam100% (1)
- Ind HW#15Document3 pagesInd HW#15studycam100% (1)
- Design and Analysis of Experiments Ind HW#15Document3 pagesDesign and Analysis of Experiments Ind HW#15studycam100% (1)
- Design of Experiments and Analysis HW#21Document4 pagesDesign of Experiments and Analysis HW#21studycam100% (1)
- Design and Analysis of Experiments HW#17Document4 pagesDesign and Analysis of Experiments HW#17studycamNo ratings yet
- Design and Analysis of Experiments HW#18Document5 pagesDesign and Analysis of Experiments HW#18studycamNo ratings yet
- Design of Experiments and Analysis HW#19Document3 pagesDesign of Experiments and Analysis HW#19studycamNo ratings yet
- Design and Analysis of Experiments Homeworks HW#20Document7 pagesDesign and Analysis of Experiments Homeworks HW#20studycam100% (1)
- Design and Analysis of Experiments HomeworksDocument3 pagesDesign and Analysis of Experiments HomeworksstudycamNo ratings yet
- Design and Analysis of ExperimentsDocument6 pagesDesign and Analysis of ExperimentsstudycamNo ratings yet
- Design and Analysis of Experiment HW#9Document4 pagesDesign and Analysis of Experiment HW#9studycamNo ratings yet
- Design and Analysis of Experiments HW#7Document7 pagesDesign and Analysis of Experiments HW#7studycamNo ratings yet
- Design and Analysis of Experiments HW#10Document3 pagesDesign and Analysis of Experiments HW#10studycamNo ratings yet
- Montgomery Design and Analysis of Experiments HomeworksDocument3 pagesMontgomery Design and Analysis of Experiments HomeworksstudycamNo ratings yet
- Design of Experiments and Analysis HomeworksDocument3 pagesDesign of Experiments and Analysis HomeworksstudycamNo ratings yet
- Design and Analysis of Experiments HW#8Document2 pagesDesign and Analysis of Experiments HW#8studycamNo ratings yet
- HW#4 For Montgomery Design and Analysis of ExperimentsDocument5 pagesHW#4 For Montgomery Design and Analysis of ExperimentsstudycamNo ratings yet
- Design and Analysis of Experiment HomeWork # 5Document8 pagesDesign and Analysis of Experiment HomeWork # 5studycamNo ratings yet
- Design of Experiments. Montgomery DoEDocument6 pagesDesign of Experiments. Montgomery DoEstudycamNo ratings yet
- Spectroscopy April 2012 Volume 27 Number 4Document62 pagesSpectroscopy April 2012 Volume 27 Number 4studycamNo ratings yet
- Physiological Barriers To Drug AbsorptionDocument41 pagesPhysiological Barriers To Drug AbsorptionstudycamNo ratings yet
- Design of Experiments. Montgomery DoEDocument6 pagesDesign of Experiments. Montgomery DoEstudycamNo ratings yet
- Spectroscopy 1012 2012Document54 pagesSpectroscopy 1012 2012studycamNo ratings yet
- Physiological Barriers To Drug AbsorptionDocument41 pagesPhysiological Barriers To Drug AbsorptionstudycamNo ratings yet
- Non Linear Least Squares Curve Fitting With Microsoft Excel SolverDocument3 pagesNon Linear Least Squares Curve Fitting With Microsoft Excel SolverstudycamNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Csec It June 2010 QaDocument16 pagesCsec It June 2010 QaLisa B Arnold50% (2)
- Kpi MR Qa QC SheDocument38 pagesKpi MR Qa QC SheAhmad Iqbal LazuardiNo ratings yet
- Phillips Et Al 2002 Positive MoodDocument11 pagesPhillips Et Al 2002 Positive MoodmechagirlNo ratings yet
- Project ManagementDocument34 pagesProject ManagementFarhan TariqNo ratings yet
- Chapter 9 Decision Making Under UncertaintyDocument15 pagesChapter 9 Decision Making Under UncertaintyjalilacastanoNo ratings yet
- DLP in English 5Document8 pagesDLP in English 5Jervyn GuiananNo ratings yet
- A Marvellous Transformation - 1020 LDocument2 pagesA Marvellous Transformation - 1020 LParth ThakkarNo ratings yet
- UTP PG Admission (Terms & Conditions) - Attachment 1 PDFDocument7 pagesUTP PG Admission (Terms & Conditions) - Attachment 1 PDFKhaleel HusainNo ratings yet
- Advances Chemical Engineering PDFDocument248 pagesAdvances Chemical Engineering PDFDaiane SantanaNo ratings yet
- Identification of The Challenges 2. Analysis 3. Possible Solutions 4. Final RecommendationDocument10 pagesIdentification of The Challenges 2. Analysis 3. Possible Solutions 4. Final RecommendationAvinash VenkatNo ratings yet
- Automatic Garbage Collector Machine: S. A. Karande S. W. Thakare S. P. Wankhede A. V. SakharkarDocument3 pagesAutomatic Garbage Collector Machine: S. A. Karande S. W. Thakare S. P. Wankhede A. V. Sakharkarpramo_dassNo ratings yet
- Principios de Aprendizaje MotorDocument22 pagesPrincipios de Aprendizaje Motorfga_vergaraNo ratings yet
- PT326-Round2 Expt3 Batch19Document6 pagesPT326-Round2 Expt3 Batch19Radhey MeenaNo ratings yet
- Adu-Yeboah Emmanuel. Mphil in Leadership, UpsaDocument146 pagesAdu-Yeboah Emmanuel. Mphil in Leadership, UpsaEmmanuel Adu-YeboahNo ratings yet
- ERPDocument9 pagesERPWindadahri PuslitkaretNo ratings yet
- HRM ThreeshortcasesDocument4 pagesHRM ThreeshortcaseskhanmohsinjamilNo ratings yet
- Magnesium Casting Technology For Structural ApplicationsDocument21 pagesMagnesium Casting Technology For Structural ApplicationsJinsoo KimNo ratings yet
- MODULI I LITERATURA Za ALAN Master Optometriju - OdtDocument3 pagesMODULI I LITERATURA Za ALAN Master Optometriju - OdtVladislav StevanovicNo ratings yet
- Schnugh V The State (Bail Appeal) Case No 92-09 (Van Niekerk & Parker, JJ) 31jan'11 PDFDocument25 pagesSchnugh V The State (Bail Appeal) Case No 92-09 (Van Niekerk & Parker, JJ) 31jan'11 PDFAndré Le RouxNo ratings yet
- Tle 6 Mam Melba (Victoria)Document2 pagesTle 6 Mam Melba (Victoria)Precious IdiosoloNo ratings yet
- Quarter 1 Science 4 Activity Sheet No. 6Document2 pagesQuarter 1 Science 4 Activity Sheet No. 6karol melendezNo ratings yet
- No Vo GADocument2 pagesNo Vo GAlongthNo ratings yet
- Reidblackman ChurchlandDocument8 pagesReidblackman ChurchlandYisroel HoffmanNo ratings yet
- Calculation of HLB ValueDocument8 pagesCalculation of HLB ValueShrutiNo ratings yet
- FIRO Element B InterpretationDocument8 pagesFIRO Element B InterpretationchinadavehkNo ratings yet
- IBPS IT Officer Model Questions Computer MIcroprocessor and Assembly Language MCQ Question BankDocument146 pagesIBPS IT Officer Model Questions Computer MIcroprocessor and Assembly Language MCQ Question BankNaveen KrishnanNo ratings yet
- Detailed Lesson Plan in MathematicsDocument5 pagesDetailed Lesson Plan in MathematicsJssy May71% (17)
- Problem Solution EssayDocument3 pagesProblem Solution Essayapi-462664573No ratings yet
- Raj Yoga ReportDocument17 pagesRaj Yoga ReportSweaty Sunny50% (2)
- Def ReadingDocument1 pageDef ReadingJembus WedutNo ratings yet