Professional Documents
Culture Documents
Oceans Regulate Earth's Climate Through Heat Absorption
Uploaded by
Ronald EnglishOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Oceans Regulate Earth's Climate Through Heat Absorption
Uploaded by
Ronald EnglishCopyright:
Available Formats
Introduction: Our Earths oceans are vast.
They play a major role in influencing our Earths climate and
weather. The oceans absorb a lot of the solar energy coming from the sun thanks to waters high heat
capacity. This regulates the temperature of the aquatic world making the temperature aquatic animals
experience stable at around one temperature. This same concept can also be brought to a global scale. The
oceans heat capacity also regulates the temperature of Earths land and directly affects the billions of people
that live upon it. While it affects coastal land more than inland, it still plays a major role in the ecosystem
around the world. We can safety assume that Earths living essence all connect one way or the other to the
oceans and lakes wellbeing. In light of the impending melting of the icecaps caused by global warming, a
large influx of fresh water will pour forth into our oceans and will alter its salinity. If the oceans change in
salinity affects the rate at which the water heats up, many environmental changes can occur. So, for this
experiment, it is set to investigate the effect of seawaters salinity to the rate of temperature increase. Four
different trials will be performed with different levels of salinity in each. Temperatures will be recorded at a
certain time interval. The experiment may end anytime after the water is boiling. Due to waters properties
such as its temperature plateau when it changes state, we can use this fact to our advantage by using the
first temperature reading in the beginning of the plateau to represent the waters boiling point. By doing
this, the rate in which temperature increases can be found.
If the experiment shows that changes in salinity can cause a shift in oceans rate of temperature increase,
further studies could be established to determine the effects on a grand scale if such an event should occur.
Question: Does changing waters salinity (simulating it using distilled water with 0%, 1%, 2%, and 3.5% of
salt) have an effect on the rate at which temperature increases?
Hypothesis:
If salinity were to be tested for its influencing factor in raising waters boiling point, then the rate at which
temperature increases at should experience a noticeable increase as more salt is added (more salt causes less
time to heat). This is because salt water has a slightly lower heat capacity compared to pure water and will
cause saltier water to boil faster.
I ndependent Variable:
Time at which the temperature readings are taken
Heat of the hot plate at 500
o
C
Salinity of each test
Dependent Variables:
The rate of temperature increase
When waters state change plateau occurs
Temperature readings
Control:
Each trial will be repeated once more for more accuracy
Not disturbing the water while heating to avoid temperature increase disruption
Pre-heated hot plate to 500
o
C to start experiment at constant temperature
Used same salt for all trials
A control of normal ocean salinity of 3.5% for better comparison and specific percentage of
salts in the other to simulate oceans dilution
Used the same distilled water for all experiments
Materials:
500mL beaker
250mL of distilled water
2.5g, 5g, 8.75g of salt
o (1%, 2%, 3.5% content respectively)
Timer
Hot plate
Thermometer
Stirring rod
Scale
Pencil and paper
Procedure:
1. Put on goggles and collect all equipment and materials listed in Materials.
2. Pour 250mL of distilled water into the 500mL beaker and measure the starting temperature using a
thermometer.
3. Preheat your hot plate to 500
o
C.
4. After preheating, Place a thermometer in the beaker and place beaker onto the hot plate and start the
timer. (See Fig. 1)
5. Every 30s (0.5 min), take a record of the temperature and record it. Try not to interfere with the water
as it may have an effect on the experiment by increasing heating rate.
6. After it begins to boil, take a few more temperature readings (temperature should be constant due to
its plateau when it changes state)
7. End the experiment and dispose of the water down the sink
8. Repeat Step 2 but this time, obtain salt and either measure 2.5g (1%), 5g (2%), or 8.75g (3.5%) of salt
using a scale. (put beaker on scale, press Tare, then measure out the salt)
9. Add salt into the beaker with distilled water. Use a stirring rod to mix the water & salt mixture until
the salt is completely dissolved in the water
10. Repeat Steps 3 to 7
11. After finishing, Repeat Steps 2 to 7 again but using a different amount of salt stated in Step 8 each
time while recording observations.
Data Collection and Processing:
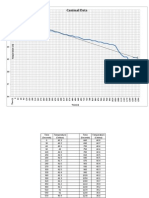
Table 1: Results of Experiment (Temperature over time)
Time
(min)
Control
A
(0.5
o
C)
Control
B
(0.5
o
C)
Trial
1A
(0.5
o
C)
Trial
1B
(0.5
o
C)
Trial
2A
(0.5
o
C)
Trial
2B
(0.5
o
C)
Trial
3A
(0.5
o
C)
Trial
3B
(0.5
o
C)
0 18.5 20 17.2 20 17 19 20 20
0.5 29.5 25.6 23.4 24 25 25.5 27 29.5
1 50 31.8 28 26.2 30 30 30 30
1.5 61.7 35.3 31.9 30.6 34 34.3 35 35.4
2 68 39.9 37.3 35.1 38 40 40 36.7
2.5 68.5 44.4 40.5 39 44 44.5 44.5 39.8
3 68.5 49.5 46.2 44.1 48 49 50.5 42.1
3.5 69.2 55.6 52.1 48.6 53.5 54 53 45.2
4 69.8 60.2 54.8 51.8 56.5 57.5 56.5 51.1
4.5 70.1 63.9 58.9 54.8 60 62 59.5 54.1
5 62.5 67 62.3 60.1 64 66 64.5 58.7
5.5 66 72.3 67.7 64 67.5 68 71.5 61.5
6 70 76.2 71 66.7 72 72 74 65.9
6.5 77.5 79.8 73.4 71.1 74.5 76 77 69.1
7 77.5 82.1 78.2 74.8 78 80.7 80 72
7.5 80.5 85.3 81 78.7 81 84 83.5 75.8
8 83 88.2 84.7 81.2 83 88 87 78.9
8.5 86 91.1 87.2 84.1 86 92 90.5 81.5
9 89.3 93.1 90.4 87.8 89 95.5 93 84
9.5 92 95.6 92 90.2 91 99 94.5 86.5
10 93.6 95.7 93.9 92.7 --- 101 94.5 90.2
10.5 95 95.7 94.3 95.5 --- 102 --- 93.1
11 95.5 95.7 --- 98 --- 102 --- 94.1
11.5 96 95.8 --- --- --- 102.1 --- 94.6
12 96 95.9 --- --- --- 103 --- 95
12.5 96 96.1 --- --- --- --- --- 95
13 96 96.1 --- --- --- --- --- 95
13.5 96 96.3 --- --- --- --- --- 95.1
14 96 96.3 --- --- --- --- --- 95.9
14.5 96 96.3 --- --- --- --- --- 96
15 96 96.3 --- --- --- --- --- 96.2
15.5 95.8 --- --- --- --- --- --- 96.8
16 96 --- --- --- --- --- --- 96
16.5 96 --- --- --- --- --- --- ---
17 95.5 --- --- --- --- --- --- ---
17.5 95.5 --- --- --- --- --- --- ---
18 95.5 --- --- --- --- --- --- ---
*Note- Trials were finished at different intervals in waters state change plateau. Therefore, the bolded
numbers in the page before are removed in analysis for accurate representation of temperature change from
room temperature to boiling temperature.
Figure 1: Temperature of 3.5% distilled salt water over time measured at 0.5 min intervals until constant
temperature is achieved. *Note- the uncertainty of 0.5
Figure 2: Temperature of 2% distilled salt water over time measured at 0.5 min intervals until constant
temperature is achieved
0
20
40
60
80
100
120
140
0 2 4 6 8 10 12
T
e
m
p
e
r
a
t
u
r
e
(
C
e
l
s
i
u
s
)
Time (mins)
Temperature of 3.5% Salt Water over Time
Control A
Control B
Linear
(Control A)
Linear
(Control B)
0
20
40
60
80
100
120
0 2 4 6 8 10 12
T
e
m
p
e
r
a
t
u
r
e
(
C
e
l
s
i
u
s
)
Time (mins)
Temperature of 2% Salt Water over Time
Trial 1A
Trial 1B
Linear
(Trial 1A)
Linear
(Trial 1B)
Figure 3: Temperature of 1% distilled salt water over time measured at 0.5 min intervals until constant
temperature is achieved.
Figure 4: Temperature of 0% distilled salt water over time measured at 0.5 min intervals until constant
temperature is achieved.
0
20
40
60
80
100
120
0 2 4 6 8 10 12
T
e
m
p
e
r
a
t
u
r
e
(
C
e
l
s
i
u
s
)
Time (mins)
Temperature of 1% Salt Water over Time
Trial 2A
Trial 2B
Linear
(Trial 2A)
Linear
(Trial 2B)
0
20
40
60
80
100
120
0 2 4 6 8 10 12
T
e
m
p
e
r
a
t
u
r
e
(
C
e
l
s
i
u
s
)
Time (mins)
Temperature of 0% Salt Water over Time
Trial 3A
Trial 3B
Linear
(Trial 3A)
Linear
(Trial 3B)
The graphs display the results in a more visual manner. Most of the graphs such as Figure 2, Figure 3, and
Figure 4 are closely equal thus confirming the results as being valid. Figure 1 shows a disturbance between
the repeated trials that could have been caused by human error as discussed under Discussion. The line of
best fit was added to each controls and trials showing the general trend of the graphs. The slope of the line of
best fit represent the rate at which temperature is increasing (Temperature over time).
ANOVA (one factor) Statistical Test
The purpose of the ANOVA Test is the compare the means of more than two treatments to detect if they are
significantly different to one another
H
0
= There is no significant difference in mean rate of temperature increase in the four salinity groups (3.5%,
2%, 1%, 0%)
H
A
= There is a significant difference in mean rate of temperature increase in the four salinity groups (3.5%,
2%, 1%, 0%)
Rate sample calculation (control treatment):
Control A: (95.5 -18.5)/2 = 7
Control B: (93.1-20)/2 = 8.122
Average Rate
(celsius/min)
7 8.122 7.343 7.091 6.947 8.2 7.45 6.736
Sample standard deviation calculation (for control treatment):
Group Mean (X
i
) Standard deviation (s) Number of values (n)
Control (3.5% salinity) 7.561111111 0.793530943 2
Trial 1 (2% salinity) 7.216883117 0.178154176 2
Trial 2 (1% salinity) 7.573684211 0.885744284 2
Trial 3 (0% salinity) 7.093181818 0.504617112 2
Sample MS
error
calculation:
Sample MS
group
calculation:
Source of
variation
Sum of squares
(SS)
Degrees of
freedom (df)
Mean squares
(MS)
F-ratio (F) Probability (P)
Group (salinity
treatments)
0.355550199 3 0.118516733
0.278762607
F < F
0.05(1) 3,4
(F<6.59)
P > 0.05
Error 1.700611635 4 0.425152909
F-distribution value is obtained from the F-distribution statistical table. As a result, our calculated F-ratio
value is less than 6.59; therefore, we fail to reject the null hypothesis. The mean rate values of all salinity
groups are not significantly different from one another to show that the samples of different salinity proves
that salinity can effect rate of temperature increase.
Discussion:
According to researcher, Stanley Zhou and his partner Geoffrey Lokke, Seawater of 35 psu has a
specific heat of 0.932 compared with 1.000 for pure water. Why does salt water reaching its boiling point
faster? Salt added into water also has an effect of lowering the freezing point and increasing the boiling point.
The research presented in this experiment does not reflect the statement above. The ANOVA test was utilized
to look for any significant difference between the 4 experimental groups. It was previously planned to use
ANOVA test to display evidence of differences for multiple groups and then utilize specific separate student
t-tests to isolate the 6 possible differences between them. The investigation was shorter than planned due to
the failure to reject the null hypothesis in the ANOVA Test. This means that the difference in the mean
values of all salinity groups is not significant enough to require further investigation. Many errors could have
led to this conclusion from the basic human error to the weakness of this specific study.
The first major error was in the test itself. The changes in temperature and the effect of salt in water
are significantly small. To prove and show a difference, large quantities of water and salt should be present.
The samples also must have a large difference such as 50 percent salt and 10 percent salt and a control of 0
percent salt comparison for any possible significant differences to be shown. The quality of the data collected
also shows faults. In Figure 1, it is shown that around the 5-minute interval, the temperature decreased and
then increased. By rationalization, heating a beaker of water and salt at a constant 500
O
C should not have
warranted any decrease in temperature while the water temperature was around 50 degrees Celsius.
There are also many unavoidable errors that were presented due to the limited time and equipment
that was supplied. The thermometers as seen in Table 1, all displayed a different starting temperature despite
using the same temperature water in the same room. The hot plate also showed inaccuracy in maintaining a
constant 500
o
C temperature. Improvements could be done by using one thermometer for all the trials to limit
the information and results from one source. This can show accurate differences better than multiple possible
different thermometers. More time would also be another factor in improving the experiment by allowing
more trials and repeats to be done. Any outlying data could be eliminated in contrast to this study where only
one additional repeat was presented, forcing the use of both data for more reliable analysis.
Human errors are unavoidable but it can be reduced to an almost insignificant factor. Some data was
caused by the moment when measuring the temperature of the water, the thermometer comes in contact with
the bottom of the beaker, a significant increase in temperature will be present. A more accurate reading
would be suspending it in the middle of the water, measuring the average temperature of all the water, not
just the heat coming from the hot plate.
The experiment can benefit from evaluating the procedure, the method, data collection, time, and
knowing the importance of these errors as stated above.
References
Byene, Robert. "Thermal Properties." Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web.
17 Jan. 2014.
Jones, James. "Stats: One-Way ANOVA." Stats: One-Way ANOVA. N.p., 21 Oct. 2012. Web. 17 Jan. 2014.
Kabakoff, Robert. "Quick-R." : ANOVA Assumptions. WebTempleteOcean, n.d. Web. 17 Jan. 2014.
Matthews, Richard. "Scientists Are Concerned about Mysterious Rise in Ocean Salinity." Global Warming Is
Real RSS. Global Health, 12 Sept. 2012. Web. 17 Jan. 2014.
Rumsey, Deborah. "Statistics." For Dummies. For Dummies, n.d. Web. 17 Jan. 2014.
Stark, Anne. "Atmospheric Warming Altering Ocean Salinity and the Water Cycle." Atmospheric Warming
Altering Ocean Salinity and the Water Cycle. Lawrence Laboratory, 4 Apr. 2012. Web. 17 Jan. 2014.
Ray Chang
IB Bio Year 1
1-1
1/12/2013
Lab Report: Salinitys effects on Rate of
Temperature Increase
You might also like
- Enhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsFrom EverandEnhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsRating: 5 out of 5 stars5/5 (1)
- Difference Between The Boiling Point of Tap and SaltwaterDocument7 pagesDifference Between The Boiling Point of Tap and SaltwaterReed GradkeNo ratings yet
- Engineering Bulletin No 1: Boiler and Furnace TestingFrom EverandEngineering Bulletin No 1: Boiler and Furnace TestingRating: 4.5 out of 5 stars4.5/5 (2)
- Measuring Moisture Content Using Thermogravimetry and Thermovolumetry MethodsDocument9 pagesMeasuring Moisture Content Using Thermogravimetry and Thermovolumetry MethodsSubhan Aristiadi RachmanNo ratings yet
- Glass Transition and Phase Transitions in Food and Biological MaterialsFrom EverandGlass Transition and Phase Transitions in Food and Biological MaterialsNo ratings yet
- Experiment 1 - Hess's LawDocument10 pagesExperiment 1 - Hess's Lawdiyana a.fNo ratings yet
- Weather Studies: The Commonwealth and International Library: Rural and Environmental Studies DivisionFrom EverandWeather Studies: The Commonwealth and International Library: Rural and Environmental Studies DivisionNo ratings yet
- Relationship Between Steam Pressure and TemperatureDocument8 pagesRelationship Between Steam Pressure and TemperatureQamarul AimanNo ratings yet
- Diffusion of Vegetable Dye in WaterDocument16 pagesDiffusion of Vegetable Dye in WaterAlejandro IvanNo ratings yet
- IB Chemistry Internal Assessment 2Document18 pagesIB Chemistry Internal Assessment 2beslisevvalNo ratings yet
- CHY113-Calorimetry FormalDocument13 pagesCHY113-Calorimetry Formalsinead5camachoNo ratings yet
- Lab Report Thermo - RealDocument12 pagesLab Report Thermo - Realazzatul amiraNo ratings yet
- Lab 2Document21 pagesLab 2Gracylla RoseNo ratings yet
- Heating Curve Lab Report - Ava MonizDocument7 pagesHeating Curve Lab Report - Ava Monizapi-533828039No ratings yet
- Thermochimica Acta: DISTAM, University of Milan, Via Celoria 2, 20133 Milan, ItalyDocument12 pagesThermochimica Acta: DISTAM, University of Milan, Via Celoria 2, 20133 Milan, ItalyStacy WilsonNo ratings yet
- Ex.3-Heat of NeutralizationDocument10 pagesEx.3-Heat of Neutralizationalia2003skNo ratings yet
- Heating Curve Lab ReportDocument11 pagesHeating Curve Lab Reportapi-460721221No ratings yet
- Perfect Gas Law Lab ReportDocument9 pagesPerfect Gas Law Lab ReportTan Zu Kuan50% (2)
- Iodine Clock Reaction RatesDocument5 pagesIodine Clock Reaction RatesRoshan RaoNo ratings yet
- How Temperature Affects The Volume of A DropDocument6 pagesHow Temperature Affects The Volume of A DropMaggie Feng100% (1)
- Marcet Boiler 1 0 AbstractDocument8 pagesMarcet Boiler 1 0 Abstractjohn rozz bbNo ratings yet
- Vapor Pressure Lab: Effect of TemperatureDocument7 pagesVapor Pressure Lab: Effect of TemperatureSuryansh KabraNo ratings yet
- Gas Absorption Report PDFDocument13 pagesGas Absorption Report PDFSaints Burner Christopher100% (1)
- Gas Absorption Lab ReportDocument12 pagesGas Absorption Lab ReportGracylla RoseNo ratings yet
- Lab ReportDocument26 pagesLab ReportMuhaimen RahmanNo ratings yet
- Titrametric Analysis Lab ReportDocument11 pagesTitrametric Analysis Lab Reportapi-546161612No ratings yet
- Thermodynamics LabDocument9 pagesThermodynamics Labmohammad. 21No ratings yet
- Lab 1Document13 pagesLab 1Jagathisswary SatthiNo ratings yet
- Chem 155 Lab 3Document11 pagesChem 155 Lab 3api-532614291No ratings yet
- Heat of Solution LabDocument4 pagesHeat of Solution LabChynna Kaye GregorioNo ratings yet
- The Best Result FinalDocument21 pagesThe Best Result FinalNana JilNo ratings yet
- Heat of FusionDocument4 pagesHeat of FusionJesse BennettNo ratings yet
- Marcet BoilerDocument7 pagesMarcet BoilerSt Oong100% (1)
- Chem Exothermic and Endothermic PractDocument7 pagesChem Exothermic and Endothermic PractMichelle Francisca Arsjad100% (1)
- Chem ActivityDocument6 pagesChem ActivityYuri MonkeyNo ratings yet
- Lab Report Finals LDocument9 pagesLab Report Finals LsofiaNo ratings yet
- Chem Lab Report 3Document8 pagesChem Lab Report 3Umar MohammedNo ratings yet
- 645 Water Conductivity PDFDocument5 pages645 Water Conductivity PDFEspañola EloiseNo ratings yet
- Calorimetry Heat of Solution Lab ReportDocument15 pagesCalorimetry Heat of Solution Lab ReportsofiaNo ratings yet
- Universidad Tecnologica Centroamericana: Cambios de FaseDocument14 pagesUniversidad Tecnologica Centroamericana: Cambios de FaseAndrea SortoNo ratings yet
- Empirical Formula Lab ReportDocument7 pagesEmpirical Formula Lab ReportSarah Marie BridgeNo ratings yet
- Bio IADocument9 pagesBio IAJuan VillanuevaNo ratings yet
- Rates of Reaction Experiment v.1.02Document6 pagesRates of Reaction Experiment v.1.02Muhammad Bilal AnwarNo ratings yet
- Keju MozarellaDocument11 pagesKeju MozarellaTaufiksyaefulmalikNo ratings yet
- Rate and Activation Energy of Iodination of AcetoneDocument5 pagesRate and Activation Energy of Iodination of AcetoneSherlock Wesley ConanNo ratings yet
- Heat of Combustion of Alcohol InvestigationDocument5 pagesHeat of Combustion of Alcohol InvestigationTuo Hundou Lee75% (4)
- Hydrometer Instruction ManualDocument12 pagesHydrometer Instruction ManualRushitNo ratings yet
- DP2 IaDocument13 pagesDP2 IaZ AlbertNo ratings yet
- Steam Pressure Curve of Saturated Steam (Marcet Boiler)Document11 pagesSteam Pressure Curve of Saturated Steam (Marcet Boiler)muhammad aqmal100% (1)
- Temperature Variation in a Marcet BoilerDocument4 pagesTemperature Variation in a Marcet BoilerMohammed Islam100% (1)
- Manaligod, Yohan I. - Experiment 2 Steam QualityDocument21 pagesManaligod, Yohan I. - Experiment 2 Steam QualityYohan ManaligodNo ratings yet
- Lab+1 4309448 4309227Document11 pagesLab+1 4309448 4309227Afwan IrfanNo ratings yet
- Experiment 3 ReportDocument6 pagesExperiment 3 ReportDolly NarisNo ratings yet
- Shell and Tube Heat Exchanger ReportDocument10 pagesShell and Tube Heat Exchanger ReportVAIBHAV MISHRANo ratings yet
- Presentación 88Document23 pagesPresentación 88Antonio HernandezNo ratings yet
- Calculating Evaporation Swimming PoolsDocument3 pagesCalculating Evaporation Swimming PoolsSyed Munawar AliNo ratings yet
- Physics IA 6Document7 pagesPhysics IA 6Aarav vermaNo ratings yet
- Laboratory Report Experiment 5 CHM476Document14 pagesLaboratory Report Experiment 5 CHM476Hazwan HamimNo ratings yet
- LR - KineticsDocument9 pagesLR - Kineticsapi-357715756No ratings yet
- Paper 3 Instruction: Answer All QuestionsDocument10 pagesPaper 3 Instruction: Answer All QuestionsNoor Hafezah Mohd MokhtiarNo ratings yet
- W3 - Intro To SDH (1) DisbowelmentDocument27 pagesW3 - Intro To SDH (1) DisbowelmentRonald EnglishNo ratings yet
- Compare Means of Treatments Using ANOVA TestDocument3 pagesCompare Means of Treatments Using ANOVA TestRonald EnglishNo ratings yet
- Social Determinants of Health TutorialDocument2 pagesSocial Determinants of Health TutorialRonald EnglishNo ratings yet
- W2 - LibraryDocument36 pagesW2 - LibraryRonald EnglishNo ratings yet
- IB History - Make Germany PayDocument2 pagesIB History - Make Germany PayRonald English100% (1)
- Brave LyricsDocument2 pagesBrave LyricsRonald EnglishNo ratings yet
- Science 10 GuideDocument49 pagesScience 10 Guideapi-283427523No ratings yet
- L6 - Interstitial Lung DiseaseDocument6 pagesL6 - Interstitial Lung DiseaseRonald EnglishNo ratings yet
- Physics 10:11 - Sample Problems and SolutionsDocument9 pagesPhysics 10:11 - Sample Problems and SolutionsRonald EnglishNo ratings yet
- Compare Means of Treatments Using ANOVA TestDocument3 pagesCompare Means of Treatments Using ANOVA TestRonald EnglishNo ratings yet
- IB Chemistry HL - Food Chemistry Option FDocument11 pagesIB Chemistry HL - Food Chemistry Option FRonald EnglishNo ratings yet
- Coming of AgeDocument4 pagesComing of AgeRonald EnglishNo ratings yet
- The Jade Peony story reveals struggles of early Chinese immigrants in CanadaDocument1 pageThe Jade Peony story reveals struggles of early Chinese immigrants in CanadaRonald EnglishNo ratings yet
- After The Macbeth TrialDocument1 pageAfter The Macbeth TrialRonald EnglishNo ratings yet
- After The Macbeth TrialDocument1 pageAfter The Macbeth TrialRonald EnglishNo ratings yet
- Relationships in "To Kill A Mockingbird" and "The House On Mango Street"Document2 pagesRelationships in "To Kill A Mockingbird" and "The House On Mango Street"Ronald EnglishNo ratings yet
- 55 Word StoryDocument1 page55 Word StoryRonald EnglishNo ratings yet
- Foods 10 Menu GormentDocument1 pageFoods 10 Menu GormentRonald EnglishNo ratings yet
- 55 Word StoryDocument1 page55 Word StoryRonald EnglishNo ratings yet
- Temperature Decay Over TimeDocument2 pagesTemperature Decay Over TimeRonald EnglishNo ratings yet
- 55 Word StoryDocument1 page55 Word StoryRonald EnglishNo ratings yet
- Comparison EssayDocument3 pagesComparison EssayRonald EnglishNo ratings yet
- Government of Mauritius BackgroundDocument2 pagesGovernment of Mauritius BackgroundRonald EnglishNo ratings yet
- Science Fair IdeasDocument1 pageScience Fair IdeasRonald EnglishNo ratings yet
- HAXO 8 Product Brochure OptDocument2 pagesHAXO 8 Product Brochure OptAnjas WidiNo ratings yet
- SPSS Data Analysis: Drowning in DataDocument47 pagesSPSS Data Analysis: Drowning in DataDdy Lee100% (4)
- 2018-Panel Data by Baun PDFDocument88 pages2018-Panel Data by Baun PDFcm_feipe100% (1)
- Bayes TheoremDocument11 pagesBayes Theoremdhiec100% (2)
- SBE - 11e Ch13b DOE and Analysis of Variance DOE ANOVADocument24 pagesSBE - 11e Ch13b DOE and Analysis of Variance DOE ANOVAjohn brownNo ratings yet
- Hengl 2009 GEOSTAT-Excelente PDFDocument293 pagesHengl 2009 GEOSTAT-Excelente PDFCharles ChavesNo ratings yet
- Destruction, Disinvestment, and Death - Economic and Human Losses Following Environmental DisasterDocument86 pagesDestruction, Disinvestment, and Death - Economic and Human Losses Following Environmental DisasterPatricia RodriguezNo ratings yet
- Assignment - ForecastingDocument1 pageAssignment - ForecastingVencint LaranNo ratings yet
- Handicaps For GlidersDocument25 pagesHandicaps For GlidersWojciech WojtaczkaNo ratings yet
- GUIDE User Manual 26.0 Department of Statistics Wisconsin-MadisonDocument247 pagesGUIDE User Manual 26.0 Department of Statistics Wisconsin-Madisonh_romeu_rsNo ratings yet
- Multicategory Logit ModelsDocument49 pagesMulticategory Logit ModelsRegine del RosarioNo ratings yet
- Lecture 13 UncertaintyDocument38 pagesLecture 13 UncertaintyTeto ScheduleNo ratings yet
- 3 Data DescriptionDocument87 pages3 Data Descriptionhsbq6s4cszNo ratings yet
- Randtests Package in RDocument15 pagesRandtests Package in RDina JankovićNo ratings yet
- Wilcoxon Test in Ordinal DataDocument15 pagesWilcoxon Test in Ordinal DataElizabeth CollinsNo ratings yet
- True/False Quiz on Time-Series Analysis TechniquesDocument1 pageTrue/False Quiz on Time-Series Analysis TechniquesBunnyNo ratings yet
- ASTM C62-12 Building BrickDocument4 pagesASTM C62-12 Building BrickJAlberto LópezNo ratings yet
- R Rec P.453 14 201908 I!!pdf eDocument24 pagesR Rec P.453 14 201908 I!!pdf emauweberNo ratings yet
- Implied VolatilityDocument15 pagesImplied VolatilityAjit SinghNo ratings yet
- Predicting Football League Tables Using Elo and Pi Ratings SystemsDocument6 pagesPredicting Football League Tables Using Elo and Pi Ratings SystemsMarián KosNo ratings yet
- Excel RegressionDocument41 pagesExcel RegressionSteve WanNo ratings yet
- Digital Manometer Accuracy 0.1Document1 pageDigital Manometer Accuracy 0.1raghavendran raghuNo ratings yet
- The Effect of Body Size On Animal AbundanceDocument8 pagesThe Effect of Body Size On Animal AbundanceChan ChibiNo ratings yet
- Idf CurveDocument7 pagesIdf CurvePATCADS STRUCTURAL SOLUTIONSNo ratings yet
- Biases in probability assessment due to heuristics and anchoringDocument5 pagesBiases in probability assessment due to heuristics and anchoringAfif UlinnuhaNo ratings yet
- ProbStat SlidesDocument70 pagesProbStat SlidesJhoanie Marie Cauan100% (2)
- 60601-1 Test Report PDFDocument167 pages60601-1 Test Report PDFVKNo ratings yet
- Preparation of DPR - Hydrological StudiesDocument30 pagesPreparation of DPR - Hydrological Studiesmathur_nkNo ratings yet
- CH - 3Document95 pagesCH - 3Gmichael GmedhnNo ratings yet
- D4867 PDFDocument5 pagesD4867 PDFAdderly Ortega100% (1)
- Cats Can Learn Too: A Simple Guide to Training Your Furry FriendFrom EverandCats Can Learn Too: A Simple Guide to Training Your Furry FriendRating: 4.5 out of 5 stars4.5/5 (55)
- Stable Relation: A Memoir of One Woman's Spirited Journey Home, by Way of the BarnFrom EverandStable Relation: A Memoir of One Woman's Spirited Journey Home, by Way of the BarnRating: 5 out of 5 stars5/5 (4)
- Mastering Parrot Behavior: A Step-by-Step Guide to Building a Strong Relationship with Your Avian FriendFrom EverandMastering Parrot Behavior: A Step-by-Step Guide to Building a Strong Relationship with Your Avian FriendRating: 4.5 out of 5 stars4.5/5 (69)
- Horse Training 101: Key Techniques for Every Horse OwnerFrom EverandHorse Training 101: Key Techniques for Every Horse OwnerRating: 4.5 out of 5 stars4.5/5 (27)
- Bird Life: A Guide to the Study of Our Common BirdsFrom EverandBird Life: A Guide to the Study of Our Common BirdsRating: 3.5 out of 5 stars3.5/5 (2)
- Your Dog Is Your Mirror: The Emotional Capacity of Our Dogs and OurselvesFrom EverandYour Dog Is Your Mirror: The Emotional Capacity of Our Dogs and OurselvesRating: 4 out of 5 stars4/5 (30)
- The Dog Listener: Learn How to Communicate with Your Dog for Willing CooperationFrom EverandThe Dog Listener: Learn How to Communicate with Your Dog for Willing CooperationRating: 4 out of 5 stars4/5 (37)
- Dog Training Journeys: A Guide to Training and Bonding with Your Mix-Breed DogFrom EverandDog Training Journeys: A Guide to Training and Bonding with Your Mix-Breed DogRating: 4.5 out of 5 stars4.5/5 (77)
- Will's Red Coat: The Story of One Old Dog Who Chose to Live AgainFrom EverandWill's Red Coat: The Story of One Old Dog Who Chose to Live AgainRating: 4.5 out of 5 stars4.5/5 (18)
- Come Back, Como: Winning the Heart of a Reluctant DogFrom EverandCome Back, Como: Winning the Heart of a Reluctant DogRating: 3.5 out of 5 stars3.5/5 (10)
- An Eagle Named Freedom: My True Story of a Remarkable FriendshipFrom EverandAn Eagle Named Freedom: My True Story of a Remarkable FriendshipNo ratings yet
- What Cats Want: An Illustrated Guide for Truly Understanding Your CatFrom EverandWhat Cats Want: An Illustrated Guide for Truly Understanding Your CatRating: 4.5 out of 5 stars4.5/5 (13)
- The Other End of the Leash: Why We Do What We Do Around DogsFrom EverandThe Other End of the Leash: Why We Do What We Do Around DogsRating: 5 out of 5 stars5/5 (63)
- Show Dog: The Charmed Life and Trying Times of a Near-Perfect PurebredFrom EverandShow Dog: The Charmed Life and Trying Times of a Near-Perfect PurebredRating: 3.5 out of 5 stars3.5/5 (13)
- The Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsFrom EverandThe Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsNo ratings yet
- Fish School 101: A Step-by-Step Guide for Fish OwnersFrom EverandFish School 101: A Step-by-Step Guide for Fish OwnersRating: 5 out of 5 stars5/5 (16)
- Roxane Gay & Everand Originals Presents: Good Girl: Notes on Dog RescueFrom EverandRoxane Gay & Everand Originals Presents: Good Girl: Notes on Dog RescueRating: 5 out of 5 stars5/5 (2)
- Edward's Menagerie: Dogs: 50 canine crochet patternsFrom EverandEdward's Menagerie: Dogs: 50 canine crochet patternsRating: 3 out of 5 stars3/5 (5)
- Puppy Training 101: How to Train a Puppy, Training Your Own Psychiatric Service Dog, A Step-By-Step Program so your Pup Will Understand You!From EverandPuppy Training 101: How to Train a Puppy, Training Your Own Psychiatric Service Dog, A Step-By-Step Program so your Pup Will Understand You!Rating: 5 out of 5 stars5/5 (85)
- Lessons from Tara: Life Advice from the World's Most Brilliant DogFrom EverandLessons from Tara: Life Advice from the World's Most Brilliant DogRating: 4.5 out of 5 stars4.5/5 (42)
- Inside of a Dog: What Dogs See, Smell, and KnowFrom EverandInside of a Dog: What Dogs See, Smell, and KnowRating: 4 out of 5 stars4/5 (390)
- The Illustrated Guide to Chickens: How to Choose Them, How to Keep ThemFrom EverandThe Illustrated Guide to Chickens: How to Choose Them, How to Keep ThemRating: 4.5 out of 5 stars4.5/5 (5)
- What It Takes to Save a Life: A Veterinarian’s Quest for Healing and HopeFrom EverandWhat It Takes to Save a Life: A Veterinarian’s Quest for Healing and HopeNo ratings yet
- The Wrong Dog: An Unlikely Tale of Unconditional LoveFrom EverandThe Wrong Dog: An Unlikely Tale of Unconditional LoveRating: 4.5 out of 5 stars4.5/5 (26)
- Arthur: The Dog Who Crossed the Jungle to Find a HomeFrom EverandArthur: The Dog Who Crossed the Jungle to Find a HomeRating: 4.5 out of 5 stars4.5/5 (18)
- Animal Reiki: Using Energy to Heal the Animals in Your LifeFrom EverandAnimal Reiki: Using Energy to Heal the Animals in Your LifeRating: 4.5 out of 5 stars4.5/5 (7)