Professional Documents
Culture Documents
Factors Affecting Internal Resistance of A Cell1
Uploaded by
SunnyChoudharyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Factors Affecting Internal Resistance of A Cell1
Uploaded by
SunnyChoudharyCopyright:
Available Formats
Factors affecting
internal resistance of a
cell
Acknowledgement
I sincerely extend my deepest gratitude to our
principal Dr. rajni for providing us with all the
facilities and kind moral support for carrying out this
project work.
I take this opportunity to give my special thanks to our
esteemed physics teacher Ms. Tripti bhagat for her
inevitable help. It is much because of his guidance
that endeavors to accomplish this project material.
Certificate
This is to certify that Sunny choudhary of class XII has
completed his physics project entitled " Factors
affecting internal resistance of a cell.
I appreciate his effort and wish him for his bright future.
Submitted to:
Ms. Tripti Bhagat
Principal
Mrs. Vijay Lakshmi (G.D salwan public school)
Acknowledgement
Certificate
Introduction
Objective
Apparatus
Theory
Procedure
Observations
Conclusions
Precautions
Sources of error
Introduction
There is a great need of batteries in our daily use
electronic appliances and the use is increasing
every day.
Thus , the batteries need to be made more
powerful so that their potential can be increased
greatly .
Thus , this project report is based on practical
analysis for the factors affecting the internal
resistance of a cell.
When the internal resistance of the cell is
decreased we can increase the potential
difference across it , and hence make it more
reliable.
Objective :-
To study the various factors on which
the internal resistance of a cell
depends.
Apparatus :-
A Potentiometer , a battery (battery eliminator) , two
way keys , a rheostat of low resistance , a galvanometer ,
a high resistance , an ammeter , a cell , a Jockey , a set
square , connecting wires , water bath , thermometer(0-
100C) , burner , tripod stand , wire gauge .
Theory :-
The internal resistance of a cell is the resistance offered
by its electrolyte to the flow of ions . The internal
resistance of a cell
is directly proportional to the distance between the
electrodes.
is inversely proportional to facing surface area of
the electrodes in electrolyte.
decreases with increase in temperature of
electrolyte.
is inversely proportional to concentration of
electrolyte.
The internal resistance of a cell is given by
r = (
)R
where
are the balancing lengths without resistance
and with resistance (shunt) , respectively and R is the
shunt resistance in parallel with the given cell.
Procedure :-
Step 1
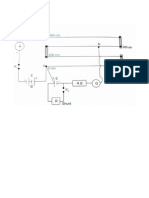
1. Draw the circuit diagram showing the scheme of
connections.
2. Clean the ends of the connecting wires with sand
paper and make tight connections according to the
circuit diagrams.
3. Tight the plugs of the resistance box.
4. Check the e.m.f. of the battery and cell and see that
e.m.f. and see that e.m.f. of the battery is more than
that of given cell ,otherwise null or balance point
will not be obtained (E' >E).
5. Take maximum current from the battery , making
rheostat resistance small.
6. To test the corrections of the connections.(insert the
plug in the key
and note the ammeter reading
.Take out 2000 ohm resistance plug from resistance
box. Place the jokey first at the end P of the wire and
then at the end Q. If the galvanometer shows
deflection in opposite direction in the two cases the
connections are correct).
7. Without inserting the plug in the key
adjust the
rheostat so that a null point is obtained on the 4th
wire of potentiometer.
8. Insert the 2000 ohm plug back in the position in
resistance box and by slightly adjusting the jockey
near the previous obtained position of null point,
obtain null point position accurately, using a set
square.
9. Measure the balancing length
between the point
and the end P of the wire.
10. Take out the 2000 ohm plug again from the
resistance box R.B. introduce plugs in the key
,as
well as in key
. Take out small resistance
(1-5 ) from the resistance box R connected in
parallel with the cell.
11. Slide the jockey along the potentiometer wire and
obtain null point.
12. Insert 2000 ohms plug back in its position in R.B.
and if necessary make further adjustment for sharp
null point.
13. Measure the balancing length
from end P.
14. Remove the plug keys at
and
.Wait for some
time and for the same value of current (as shown by
ammeter) repeat the steps 7 to 13.
15. Repeat the observations for diffrent values of R
repeating each observation twice.
16. Calculate the internal resistance of cell by using the
above relation for r.
Step 2
To see the effect of distance between the
electrodes on internal resistances keeping the
other factors constant ,vary separation between
electrodes and measure internal resistance in
each case.
Step 3
To see the effect of the temperature of electrolyte
on internal resistance by keeping other factors
constant.
Keep primary cells in water bath to heat
the electrolyte. Determine the internal
resistance at various temperatures.
Step 4
To see the effect of concentration (nature) of
electrolyte on internal resistance by :-
Keeping the other factors constant , decrease
concentration of electrolyte by adding the distilled
water and determine internal resistance of cell in
each case .
Diagram:-
Observations :-
S.No
.
Ammete
r
Reading
Pos. of null
point ( cm )
Shunt
Resistanc
e
r=(
)
R
( A ) Wit
h R
(l
1
)
Withou
t R ( l
2
)
R ( )
1.
2.
3.
Table for effect of separation between electrodes :-
Table for effect of temperature :-
S.No. Temper-
ature
l
1
l
2
Resist
ance
r=(
)R
Tr
(T) C (cm) (cm) R () () (K)
1.
2.
3.
S.No
.
Separatio
n between
Balancin
g length
Balancin
g length
r=(
)
R
r/
d
Electrodes
-d (cm)
(cm) ( l
1
)
(cm) (l
2
) ( )
1.
2.
3.
Conclusions :-
1. The Electromotive Force of the cell is constant and is
equal to E = 0.98
Volt
2. The internal resistance of a cell is directly
proportional to the separation between the
electrodes.
3. The internal resistance of a cell is inversely
proportional to the area of the electrodes dipped in
electrolyte.
4. The internal resistance of a cell is inversely
proportional to the temperature of electrolytes.
5. The internal resistance of a cell is inversely
proportional to the concentration of the electrolyte.
Precautions :-
1. The connections should be neat , clean and tight.
2. The plugs should be introduced in the keys only
when the observations are to be taken.
3. The positive polls of the battery E and cells E1 and E2
should , all be connected to the terminal at the zero
of the wires.
4. The jockey key should not be rubbed along the wire.
It should touch the wire gently.
5. The ammeter reading should remain constant for a
particular set of observation. If necessary , adjust
the rheostat for this purpose.
6. The e.m.f. of the battery should be greater than the
e.m.f.'s of the either of the two cells.
7. Some high resistance plug should always be taken
out from resistance box before the jockey is moved
along the wire.
8. The e.m.f. of the battery should be greater than that
of the cell.
9. For one set of observation the ammeter reading
should remain constant.
10. Current should be passed for short time only , while
finding the null point.
11. Rheostat should be adjusted so that initial null
point lies on last wire of the potentiometer.
12. Cell should not be disturbed during experiment.
13. Jockey should not be rubbed against the
potentiometer wire.
Sources of error :-
1. The auxiliary battery may not be fully charged.
2. The potentiometer wire may not be of uniform
cross-section and material density throughout its
length.
3. End resistances may not be zero.
Bibliography
www.Google.com
www.Wikipedia.com
www.scribd.com
You might also like
- To Study The Factors On Which The Internal Resistance of A Cell DeoendsDocument17 pagesTo Study The Factors On Which The Internal Resistance of A Cell DeoendsSakshi GodaraNo ratings yet
- Physics Investigatory Project: Factors Affecting Internal Resistance of A CellDocument18 pagesPhysics Investigatory Project: Factors Affecting Internal Resistance of A Cellutkarsh thakur78% (9)
- Project On Factors Affecting Internal Resistance of CellDocument11 pagesProject On Factors Affecting Internal Resistance of Cellprateekgoel99955% (47)
- To Study The Factors On Which The Internal Resistance of A Cell DependsDocument18 pagesTo Study The Factors On Which The Internal Resistance of A Cell DependsWashiul Islam100% (1)
- Internal Resistance of Cell ProjectDocument12 pagesInternal Resistance of Cell Projectmandeep singh100% (1)
- NAME: Raghunath Marandi Class: Xii-C TOPIC: To Study Various Factors On Which The Internal Resistance / EMF of A Cell DependsDocument20 pagesNAME: Raghunath Marandi Class: Xii-C TOPIC: To Study Various Factors On Which The Internal Resistance / EMF of A Cell DependsSwaraj Patel100% (3)
- Kendriya Vidyalaya Ambarnath Physics Investigatory ProjectDocument22 pagesKendriya Vidyalaya Ambarnath Physics Investigatory ProjectROHIT SIHRA100% (2)
- Physicsinvestigatoryproject 170908150740Document20 pagesPhysicsinvestigatoryproject 170908150740Nx GamingYTNo ratings yet
- To Study The Factors On Which The Internal Resistance of A Cell Depends.Document16 pagesTo Study The Factors On Which The Internal Resistance of A Cell Depends.Dhananjay63% (101)
- Project On Factors Affecting Internal Resistance of CellDocument23 pagesProject On Factors Affecting Internal Resistance of Cellhmoedmhop46% (13)
- Physics ProjectDocument18 pagesPhysics ProjectPrajesh Biswas100% (1)
- Project Rahul (Internal Resistance)Document13 pagesProject Rahul (Internal Resistance)Ricky Kumar72% (18)
- Sotyo Proko Blic School: TopicDocument15 pagesSotyo Proko Blic School: TopicYahyouknowme100% (1)
- Factors On Which The Internal Resistance - Emf of A Cell DependsDocument20 pagesFactors On Which The Internal Resistance - Emf of A Cell DependsSabita Talukdar100% (1)
- Physics Investigatory ProjectDocument17 pagesPhysics Investigatory ProjectSubhasish BeheraNo ratings yet
- Chemistry InvestigatoryDocument23 pagesChemistry InvestigatoryAditi Goyal100% (3)
- Physics Investigatory ProjectDocument27 pagesPhysics Investigatory ProjectRashmit BhanderiNo ratings yet
- To Study Various Factors On Which The Internal Resistance/emf of A Cell DependsDocument12 pagesTo Study Various Factors On Which The Internal Resistance/emf of A Cell DependsRudraunsh Yadav80% (15)
- Physics Project - Internal Resistance of A Primary Cell-3Document5 pagesPhysics Project - Internal Resistance of A Primary Cell-3Aaryan Shroff100% (2)
- Physics Project (Investigatory Project 9)Document8 pagesPhysics Project (Investigatory Project 9)Ashu58% (19)
- Factors On Which Self Inductance of CoilDocument7 pagesFactors On Which Self Inductance of CoilShikhar BajpaiNo ratings yet
- 1 To Study Various Factors On Which The Internal resistanceEMF of A Cell Depends.Document11 pages1 To Study Various Factors On Which The Internal resistanceEMF of A Cell Depends.shiva nayak100% (4)
- Physics Investigatory ProjectDocument8 pagesPhysics Investigatory Projectharry potter33% (3)
- Study of Constituents of Alloys - ChemistryInvestigatoryProjectDocument17 pagesStudy of Constituents of Alloys - ChemistryInvestigatoryProjectAnirudh Aurange75% (4)
- Chemistry Investigatory ProjectDocument11 pagesChemistry Investigatory Project11AN Kanishk Kumar S100% (3)
- Physics Investigatory ProjectDocument12 pagesPhysics Investigatory ProjectAyushi ShakyaNo ratings yet
- Project Report On Low Cost Emergency LightDocument3 pagesProject Report On Low Cost Emergency LightyashsviNo ratings yet
- To Assemble A Household Circuit Comprising Three Bulbs, Three (On/off) Switches A Fuse and A Power Source.Document4 pagesTo Assemble A Household Circuit Comprising Three Bulbs, Three (On/off) Switches A Fuse and A Power Source.Study Material100% (1)
- Biology Project Class 12Document21 pagesBiology Project Class 12sri madhurita100% (1)
- Chem ProjectDocument20 pagesChem ProjectAkshat dixit100% (1)
- Physics Investigatory CLASS 12Document13 pagesPhysics Investigatory CLASS 12Hacker246 Gaming100% (2)
- To Study Various Factors On Which The Internal Resistance Emf of A Cell DependsDocument17 pagesTo Study Various Factors On Which The Internal Resistance Emf of A Cell Dependsprashik vigheNo ratings yet
- Chemistry Project - ConductivityDocument19 pagesChemistry Project - ConductivityPankaj Gill67% (3)
- Chemistry Project (XII)Document21 pagesChemistry Project (XII)Anwesha Kar, XII B, Roll No:110% (4)
- Self Conductance of A CoilDocument15 pagesSelf Conductance of A CoilPranathiNo ratings yet
- To Assemble A Household Circuit Comprising Three Bulbs, Three (Onoff) Switches, A Fuse & A Power SourceDocument2 pagesTo Assemble A Household Circuit Comprising Three Bulbs, Three (Onoff) Switches, A Fuse & A Power Sourceaff bearNo ratings yet
- Factors On Which Self Inductance Depends.Document9 pagesFactors On Which Self Inductance Depends.Soumya Ramaiya100% (3)
- Activity Notes BookDocument12 pagesActivity Notes Bookkaran singh class 11 aNo ratings yet
- Chemistry Investigatory ProjectDocument14 pagesChemistry Investigatory ProjectKhanna Kiara100% (2)
- Biology Investigatory ProjectDocument14 pagesBiology Investigatory ProjectPulkit0% (1)
- Chemistry ProjectDocument15 pagesChemistry ProjectArjun Chauhan50% (2)
- XII Physics Investigatory Project To Study The Factor On Which The Self-Inductance of A Coil Depends (2) FinalDocument15 pagesXII Physics Investigatory Project To Study The Factor On Which The Self-Inductance of A Coil Depends (2) FinalMehul Ananthakumar100% (3)
- Chemistry Investigatory ProjectDocument21 pagesChemistry Investigatory ProjectDivyanshu Gupta33% (3)
- Chemistry Investigatory Project: Study The Change in E.M.F of A Daniel CellDocument20 pagesChemistry Investigatory Project: Study The Change in E.M.F of A Daniel CellrahuhlNo ratings yet
- Water Concentration and TextureDocument8 pagesWater Concentration and TextureFatin Izzati Zainal Abidin100% (2)
- Ohms Law ProjectDocument7 pagesOhms Law Projectapi-2641987250% (4)
- Physics Investigatory Project Class 12 CbseDocument17 pagesPhysics Investigatory Project Class 12 Cbseanon_930629608No ratings yet
- Chemistry Investigatory ProjectDocument13 pagesChemistry Investigatory ProjectAshrayee Wasnik100% (1)
- Ragul PhyDocument15 pagesRagul Phyragul100% (2)
- Hariom Kumar RoyDocument24 pagesHariom Kumar RoyRajeev Kumar Roy0% (1)
- Physics Investigatory Project: Common Base TransistorDocument13 pagesPhysics Investigatory Project: Common Base TransistorJoydeep Naskar64% (167)
- PHYSICS Project Class 12Document16 pagesPHYSICS Project Class 12mihir khabiya100% (1)
- Deepti Thakur-To-Study-The-Factors-On-Which-The-Internal-Resistance-Of-A-Cell-DependsDocument21 pagesDeepti Thakur-To-Study-The-Factors-On-Which-The-Internal-Resistance-Of-A-Cell-Dependsdhruv asatiNo ratings yet
- Physics ProjectDocument11 pagesPhysics ProjectUmeshSwaroop67% (3)
- Factors Affecting Internal Resistance of A CellDocument16 pagesFactors Affecting Internal Resistance of A CellChikkumol BabuNo ratings yet
- PhysicsDocument21 pagesPhysicsAachal GayreNo ratings yet
- Project On Factors Affecting Internal Resistance of CellDocument11 pagesProject On Factors Affecting Internal Resistance of CellVeenaGupta60% (5)
- Wa0001Document18 pagesWa0001Hariom HarshNo ratings yet
- PROJECT1 Project On Factors Affecting Internal Resistance of CellDocument12 pagesPROJECT1 Project On Factors Affecting Internal Resistance of CellrahulNo ratings yet
- Sloan Casebook 2015Document46 pagesSloan Casebook 2015Tharitha Murage100% (2)
- CaseBook WSBDocument131 pagesCaseBook WSBSunnyChoudhary100% (1)
- SCM Notes PDFDocument48 pagesSCM Notes PDFSunnyChoudharyNo ratings yet
- Guesstimate in The Indian ContextDocument13 pagesGuesstimate in The Indian ContextAnant Kumar100% (9)
- 32 Project ManagementDocument252 pages32 Project ManagementAjay MalikNo ratings yet
- Guesstimate in The Indian ContextDocument13 pagesGuesstimate in The Indian ContextAnant Kumar100% (9)
- Guide Case InterviewsDocument2 pagesGuide Case InterviewsLucky YohNo ratings yet
- Introduction To Project ManagementDocument72 pagesIntroduction To Project Managementparul vyasNo ratings yet
- Michael E. Porters Five Forces Model Ebook From WikipediaDocument27 pagesMichael E. Porters Five Forces Model Ebook From WikipediaD. Mumtaz.SNo ratings yet
- Vittore Cossalter Motorcycle Dynamics BDocument405 pagesVittore Cossalter Motorcycle Dynamics BAsad Ali100% (8)
- Principles by IUCG - Mastering The Case Interview PRINT FILEDocument8 pagesPrinciples by IUCG - Mastering The Case Interview PRINT FILESunnyChoudharyNo ratings yet
- Michael E. Porters Five Forces Model Ebook From WikipediaDocument27 pagesMichael E. Porters Five Forces Model Ebook From WikipediaD. Mumtaz.SNo ratings yet
- (WWW - Cgaspirants.com) Operations Research - D S HiraDocument1,512 pages(WWW - Cgaspirants.com) Operations Research - D S HiraEr Rohit Kumar100% (6)
- Tribology NotesDocument17 pagesTribology NotesSunnyChoudharyNo ratings yet
- Economics For Engineering Stude - Singh SeemaDocument357 pagesEconomics For Engineering Stude - Singh SeemaSunnyChoudhary100% (1)
- R.K. Bansal-A Textbook of Fluid Mechanics and Hydraulic Machines 9th Revised Edition SI Units (Chp.1-11) - Laxmi Publications (2010) PDFDocument578 pagesR.K. Bansal-A Textbook of Fluid Mechanics and Hydraulic Machines 9th Revised Edition SI Units (Chp.1-11) - Laxmi Publications (2010) PDFgayatri_vyas178% (18)
- FM 20180307091355Document9 pagesFM 20180307091355SunnyChoudharyNo ratings yet
- Machine Design Data BookDocument19 pagesMachine Design Data BookSunnyChoudharyNo ratings yet
- Nptel 2018 BookletDocument322 pagesNptel 2018 BookletAdhiraj C Choudhary0% (1)
- A Text Book of Fluid Mechanics and Hydraulic Machines - R. K. BansalDocument1,129 pagesA Text Book of Fluid Mechanics and Hydraulic Machines - R. K. BansalVignesh Vicky81% (16)
- On The Face of ItDocument3 pagesOn The Face of ItSunnyChoudharyNo ratings yet
- Marketing MixDocument21 pagesMarketing MixSunnyChoudharyNo ratings yet
- PROGRAM #13 Aim:Implementing Structure //program To Read Employee Data and Print It On ScreenDocument5 pagesPROGRAM #13 Aim:Implementing Structure //program To Read Employee Data and Print It On ScreenSunnyChoudharyNo ratings yet
- The Invisible Man 17 - 18Document2 pagesThe Invisible Man 17 - 18SunnyChoudharyNo ratings yet
- Periodic Properties PDFDocument31 pagesPeriodic Properties PDFDeependraNo ratings yet
- Chem Project AntacidDocument20 pagesChem Project AntacidSunnyChoudharyNo ratings yet
- Computer Science ProjectDocument40 pagesComputer Science ProjectSunnyChoudhary100% (2)
- Computer Science ProjectDocument40 pagesComputer Science ProjectSunnyChoudhary100% (2)
- Transport of Mineral Nutrients: Bhawna MadanDocument41 pagesTransport of Mineral Nutrients: Bhawna MadanSunnyChoudharyNo ratings yet
- Solid StateDocument14 pagesSolid StateSunnyChoudharyNo ratings yet
- 106資優特訓班Document96 pages106資優特訓班utaNo ratings yet
- Question Bank Centroid&MIDocument13 pagesQuestion Bank Centroid&MIrubyNo ratings yet
- Solutions # 8: Department of Physics IIT Kanpur, Semester II, 2022-23Document9 pagesSolutions # 8: Department of Physics IIT Kanpur, Semester II, 2022-23darshan sethiaNo ratings yet
- Fender Analysis PDFDocument242 pagesFender Analysis PDFjay climacoNo ratings yet
- Maxwell's Equations: and God Said, "Let There Be Light" and There Was Light ! - GenesisDocument13 pagesMaxwell's Equations: and God Said, "Let There Be Light" and There Was Light ! - GenesisYash KalaNo ratings yet
- 2002, Hirano Et Al.Document9 pages2002, Hirano Et Al.sumitNo ratings yet
- บทที่ 3 thermodynamicsDocument11 pagesบทที่ 3 thermodynamicsAnonymous nveiFINo ratings yet
- Lec-12 - Distillation of Non-Ideal SystemDocument28 pagesLec-12 - Distillation of Non-Ideal SystemsamrahamidNo ratings yet
- Physics Chapter 11Document18 pagesPhysics Chapter 11cryptachrisNo ratings yet
- 7 - Circular Motion and RotationDocument38 pages7 - Circular Motion and RotationImran SitompulNo ratings yet
- Physics 9702 Paper 1 - Current of ElectricityDocument41 pagesPhysics 9702 Paper 1 - Current of ElectricitySalman ShahidNo ratings yet
- Aqwa Theory ManualDocument200 pagesAqwa Theory ManualV CafNo ratings yet
- The Nature and Perception of SoundDocument21 pagesThe Nature and Perception of Soundsheila louiseNo ratings yet
- Quantum Mechanics NotesDocument42 pagesQuantum Mechanics Notessimonm_92No ratings yet
- General Properties of HydrogelsDocument15 pagesGeneral Properties of HydrogelsJimmy NelsonNo ratings yet
- Simple Harmonic MotionDocument4 pagesSimple Harmonic MotionNuyara VithanageNo ratings yet
- Inductive and Capacitive SensorsDocument57 pagesInductive and Capacitive SensorsseenuNo ratings yet
- Nonlinear Systems and Control Lecture # 7 Stability of Equilibrium Points Basic Concepts & LinearizationDocument19 pagesNonlinear Systems and Control Lecture # 7 Stability of Equilibrium Points Basic Concepts & LinearizationLasandu WanniarachchiNo ratings yet
- Science: Quarter 2 - Module 1: Different Forms of EM WavesDocument25 pagesScience: Quarter 2 - Module 1: Different Forms of EM WavesMercy Mangaoil100% (3)
- Grade 8 - III - SummativeDocument3 pagesGrade 8 - III - Summativemenchie ismael100% (4)
- F 5 C 2Document14 pagesF 5 C 2jalrizal7No ratings yet
- Experimental Investigation of A Photovoltaic-Powered Solar Cassava DryerDocument7 pagesExperimental Investigation of A Photovoltaic-Powered Solar Cassava DryerJuan CifuentesNo ratings yet
- Physics Practice Final With SolutionDocument27 pagesPhysics Practice Final With SolutionKim Jong UnNo ratings yet
- George Salvan Architectural Utilities 3 Lighting and Acoustics PDFDocument530 pagesGeorge Salvan Architectural Utilities 3 Lighting and Acoustics PDFLawrence Ibasco89% (9)
- Fundamentals of Applied Dynamics Revised PrintingDocument1 pageFundamentals of Applied Dynamics Revised PrintingMochamad Safarudin0% (3)
- TOS - 3rd - Science 7Document2 pagesTOS - 3rd - Science 7Ley CanasaNo ratings yet
- Slaughter 1305, 2503, 2510, 2550 OpsDocument42 pagesSlaughter 1305, 2503, 2510, 2550 OpsFabio TempelNo ratings yet
- Ferranti EffectDocument2 pagesFerranti EffectVaibhav Singh RajputNo ratings yet
- Energy and Exergy Analysis of A Simple Gas Turbine Cycle With Wet CompressionDocument11 pagesEnergy and Exergy Analysis of A Simple Gas Turbine Cycle With Wet CompressionKrishnaDuttPandeyKdpNo ratings yet
- Proposed Two Storey Residence ReportDocument27 pagesProposed Two Storey Residence ReportchorgedNo ratings yet