Professional Documents
Culture Documents
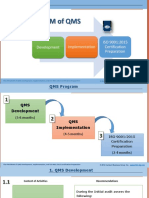
QMS To ISO 9001
Uploaded by
ManInTheBushOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
QMS To ISO 9001
Uploaded by
ManInTheBushCopyright:
Available Formats
Quality policy
Quality objectives
communicating to the organization the importance of meeting customer as well as statutory and regulatory requirements,
includes a commitment to comply with requirements and continually improve the effectiveness of the quality management system,
Measurable
Scope
Quality Manual
Reference to the Procedures of the QMS
Description of the interaction
between the QMS procedures
a) to approve documents for adequacy prior to issue,
b) to review and update as necessary and re-approve documents,
c) to ensure that changes and the current revision status of documents are identified,
d) to ensure that relevant versions of applicable documents are available at points of use,
Document Control
e) to ensure that documents remain legible and readily identifiable,
f) to ensure that documents of external origin determined by the organization to be necessary for the planning
and operation of the quality management system are identified and their distribution controlled, and
g) to prevent the unintended use of obsolete documents, and to apply suitable identification to them if they
are retained for any purpose.
identification,
storage
protection
retention
Record Control
retrieval
and disposition of records.
Records shall remain legible, readily identifiable and retrievable.
provides a framework for establishing and reviewing quality objectives,
results of audits,
customer feedback,
process performance and product conformity,
status of preventive and corrective actions,
QMS Review
follow-up actions from previous
management reviews,
changes that could affect the quality
management system, and
recommendations for improvement.
ensuring that processes needed for the quality management system are established, implemented and maintained,
QMS Audit
reporting to top management on the performance of the quality management system and any need for improvement, and
ensuring the promotion of awareness of customer requirements throughout the organization.
QA Induction
Employment
Minimum qualification
Training Records
Scope: 7.2.1
Scope Review: 7.2.2
Customer Communication: 7.2.3
Inputs: 7.3.2
Outputs: 7.3.3
Review: 7.3.4
Design and development planning: 7.3.1
Verification: 7.3.5
Validation: 7.3.6
Change: 7.3.7
Procedures
Selection criteria
Purchasing Information: 7.4.2
Purchasing: 7.4
a) requirements for approval of product, procedures, processes and equipment,
b) requirements for qualification of personnel, and
c) quality management system requirements.
Verification of purchased product
Quality Management System
Vendor evaluation
DeR/NCR
a) the availability of information that describes the characteristics of the product,
Procedures and Records
required by ISO 9001
b) the availability of work instructions, as necessary,
c) the use of suitable equipment,
Execution
Control: 7.5.1
d) the availability and use of monitoring and measuring equipment,
e) the implementation of monitoring and measurement, and
f) the implementation of product release, delivery and post-delivery activities.
Release
a) defined criteria for review and approval of the processes,
Mandatory Plans
Production and Service Provision: 7.5
Validation: 7.5.2 (where output cannot
be verified or monitored)
b) approval of equipment and qualification of personnel,
c) use of specific methods and procedures,
d) requirements for records
e) revalidation
Identification and traceability: 7.5.3
identify, verify, protect and safeguard
customer property
Customer Property: 7.5.4
Preservation of Product: 7.5.5
Control of Monitoring and Measuring
equipment: 7.6
Customer Satisfaction: 8.2.1
Internal Audit of QMS: 8.2.2
Monitoring and Measurement: 8.2
Monitoring and measuring of QMS
processes: 8.2.3
Monitoring and measuring of QC
processes: 8.2.4
a) by taking action to eliminate the detected nonconformity;
Measurement, Analysis and
Improvement: 8
Control of Nonconforming product: 8.3
b) by authorizing its use, release or acceptance under concession by a relevant authority
and, where applicable, by the customer;
c) by taking action to preclude its original intended use or application;
d) by taking action appropriate to the effects, or potential effects, of the nonconformity when
nonconforming product is detected after delivery or use has started.
Analysis: 8.4
Continual: 8.5.1
Improvement: 8.5
Corrective: 8.5.2
Preventive: 8.5.3
improvement of the effectiveness of the quality management system and its processes,
QMS Review
improvement of product related to customer requirements, and
resource needs.
TRM
Defines responsibilities and authorities
Management representative
QMS Audit
Site plan
Asset lists
Infrastructure
Documents
Supporting Systems
Off-site infrastructure? Subs?
Work environment controls
a) be calibrated or verified, or both, at specified intervals, or prior to use, against measurement standards traceable to international or
national measurement standards; where no such standards exist, the basis used for calibration or verification shall be recorded;
b) be adjusted or re-adjusted as necessary;
Monitoring and measuring
Schedule
c) have identification in order to determine its calibration status;
d) be safeguarded from adjustments that would invalidate the measurement result;
e) be protected from damage and deterioration during handling, maintenance and storage.
- - Mindjet
Documents and Records required
by BAM International
You might also like
- DFC 4.1Document1 pageDFC 4.1Muhammad WaqasNo ratings yet
- 5.6 Management Review: 5.6.1 GeneralDocument1 page5.6 Management Review: 5.6.1 GeneralTina MillerNo ratings yet
- Tools ISO 9001Document3 pagesTools ISO 9001Miftakhul Nurdianto100% (1)
- Control of Monitoring and Measuring EquipmentDocument3 pagesControl of Monitoring and Measuring EquipmentLinda Setya WatiNo ratings yet
- Process Check ListDocument5 pagesProcess Check Listapi-338883409No ratings yet
- Iso 9001:2000 Gap Checklist: 4.0 Quality Management System 4.1 General RequirementsDocument11 pagesIso 9001:2000 Gap Checklist: 4.0 Quality Management System 4.1 General Requirementscover filterNo ratings yet
- Iso and Quality Ob 3 RAWDocument25 pagesIso and Quality Ob 3 RAWsagarsam001No ratings yet
- MFG Procedure ManualDocument46 pagesMFG Procedure ManualAdinanNo ratings yet
- QMS Core Processes Manual ISO 9001 2015Document122 pagesQMS Core Processes Manual ISO 9001 2015rj Turno100% (1)
- ISO 9001 - 2015 Audit Check ListDocument10 pagesISO 9001 - 2015 Audit Check Listsandeep KumarNo ratings yet
- Comparative Matrix QMS 2015 and QMS 2008Document21 pagesComparative Matrix QMS 2015 and QMS 2008lenvfNo ratings yet
- Quality DashboardDocument64 pagesQuality DashboardEkane NgemeNo ratings yet
- What Is A Document?: Tips On ISO 9001 Quality Management System DocumentationDocument5 pagesWhat Is A Document?: Tips On ISO 9001 Quality Management System DocumentationMohammad Jaid AlamNo ratings yet
- Star Ecn Form 2012Document4 pagesStar Ecn Form 2012BERK YENİLMEZNo ratings yet
- 2.06 SIPOC DiagramDocument4 pages2.06 SIPOC DiagramJulioRomeroNo ratings yet
- List of Documents ISO 9001 Documentation Toolkit enDocument2 pagesList of Documents ISO 9001 Documentation Toolkit enocom706No ratings yet
- 9001 Audit Checklist - Quality Planning and DesignDocument14 pages9001 Audit Checklist - Quality Planning and DesignAmer RahmahNo ratings yet
- The Program of QmsDocument16 pagesThe Program of QmsHamza Sharif AdamNo ratings yet
- Managing The Process Flow With ISO9000 FamilyDocument6 pagesManaging The Process Flow With ISO9000 FamilyVC100% (1)
- How To Conduct Management ReviewDocument8 pagesHow To Conduct Management ReviewS Seetharaman100% (1)
- How To Use The Audit Program Manager: List The Processes/functional AreasDocument25 pagesHow To Use The Audit Program Manager: List The Processes/functional AreasAliNo ratings yet
- List of Contents: Process-And Product AuditDocument6 pagesList of Contents: Process-And Product AuditiresendizNo ratings yet
- ISO-TS, Core Tools, and Other Resources MatrixDocument4 pagesISO-TS, Core Tools, and Other Resources MatrixToni HimawanNo ratings yet
- Summary of Iso 90091Document3 pagesSummary of Iso 90091Ihuhwa Marta TauNo ratings yet
- Iso 9001 Implementation WorkplanDocument9 pagesIso 9001 Implementation WorkplanSANo ratings yet
- Management ChecklistDocument4 pagesManagement ChecklistssslucysssNo ratings yet
- Iso9001 2015 Quality Manual Template 1 1024Document1 pageIso9001 2015 Quality Manual Template 1 1024Adhi GunantoNo ratings yet
- Matrice de Conformite - Norme API q1Document30 pagesMatrice de Conformite - Norme API q1Mohamed BencharifNo ratings yet
- EEN Audit Format Sample GuideDocument2 pagesEEN Audit Format Sample GuideDuane SchumacherNo ratings yet
- Audit Program Manager 9k, 14k - Risk Based (14jul2017)Document62 pagesAudit Program Manager 9k, 14k - Risk Based (14jul2017)AliNo ratings yet
- Guidelines For Supplier Audit: 1.1 Process Capability-Capabilitatea ProcesuluiDocument15 pagesGuidelines For Supplier Audit: 1.1 Process Capability-Capabilitatea ProcesuluiAdi SavaNo ratings yet
- What Is The ISO 9001:2008 Audit Checklist?Document38 pagesWhat Is The ISO 9001:2008 Audit Checklist?John SoaresNo ratings yet
- ISO 9001 Implementation PlanDocument2 pagesISO 9001 Implementation PlanbevarsiNo ratings yet
- Dash Board Sheet With Base DataDocument7 pagesDash Board Sheet With Base Datavivek1312No ratings yet
- Construction Quality AssuranceDocument8 pagesConstruction Quality AssuranceMalek AlhussienNo ratings yet
- 质量过程审核 Quality Process Audit: 修订履历 Revision HistoryDocument42 pages质量过程审核 Quality Process Audit: 修订履历 Revision HistoryphamtienkhangNo ratings yet
- Session-2 & 3 Quality - Evolution of QualityDocument20 pagesSession-2 & 3 Quality - Evolution of Qualitymatten yahyaNo ratings yet
- ISO 9001 Required DocumentationDocument3 pagesISO 9001 Required Documentationdnmule100% (1)
- QMS-SOP-F13 - Performance EvaluationDocument3 pagesQMS-SOP-F13 - Performance EvaluationPINTU RAJNo ratings yet
- QMS, EMS ChecklistDocument9 pagesQMS, EMS Checklistgayathrisrk001No ratings yet
- ISO 9001:2015 Preparation Steps: No Activities Deadline Person in Charges 1 CommitmentDocument3 pagesISO 9001:2015 Preparation Steps: No Activities Deadline Person in Charges 1 CommitmentRhisnaErdyDianaNo ratings yet
- ISO 9001 2015 Transition Checklist C 01 Rev ADocument4 pagesISO 9001 2015 Transition Checklist C 01 Rev APlant Head PrasadNo ratings yet
- Audit Evidence Evaluation Comments: Internal Audit Check List Mr/Cip/Training/Customer ComplaintsDocument3 pagesAudit Evidence Evaluation Comments: Internal Audit Check List Mr/Cip/Training/Customer ComplaintsganrashNo ratings yet
- Form - Management Review Meeting MinutesDocument6 pagesForm - Management Review Meeting Minutesmgamal1080100% (1)
- Why ISO 9001:2015? Awareness Presentation: Subtitle or PresenterDocument15 pagesWhy ISO 9001:2015? Awareness Presentation: Subtitle or PresenterMarusia TirmicanNo ratings yet
- Draft in Qa Form 01 EcnDocument2 pagesDraft in Qa Form 01 EcnSuraj RawatNo ratings yet
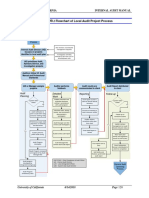
- 6000 Appendix 6000.: 2 Flowchart of Local Audit Project ProcessDocument1 page6000 Appendix 6000.: 2 Flowchart of Local Audit Project ProcessNiken RindasariNo ratings yet
- ISO 9001:2008 ISO/TS 16949:2009 Added Requirement: 0.5 Goal of This Technical SpecificationDocument52 pagesISO 9001:2008 ISO/TS 16949:2009 Added Requirement: 0.5 Goal of This Technical Specificationsupady5751No ratings yet
- 9100 Key Changes PresentationDocument1 page9100 Key Changes PresentationrakshithNo ratings yet
- Management Review Meeting FormatDocument2 pagesManagement Review Meeting FormatThusitha LakpriyaNo ratings yet
- 7 Steps To Business Process MappingDocument1 page7 Steps To Business Process MappingCreative Technology and DesignNo ratings yet
- Maintenance ManagementDocument39 pagesMaintenance ManagementDurbar DasguptaNo ratings yet
- 2-12-2020 Final QUALITY MANAGEMENT SYSTEM OrientationDocument92 pages2-12-2020 Final QUALITY MANAGEMENT SYSTEM Orientationdrmc petuNo ratings yet
- Iso 9001:2008 Quality Management System Implementation Plan: PlanningDocument1 pageIso 9001:2008 Quality Management System Implementation Plan: PlanningKhirullah Abdul HamidNo ratings yet
- Management & Control of QualityDocument2 pagesManagement & Control of QualityDeepak Singh NegiNo ratings yet
- ISO 9001 Internal Audit ChecklistDocument14 pagesISO 9001 Internal Audit ChecklistEsterNTNo ratings yet
- COMP-OPP-02 Procedure For Control and Validation of Service ProvisionDocument6 pagesCOMP-OPP-02 Procedure For Control and Validation of Service ProvisionISODCC DSPINo ratings yet
- ISO 9001-2015 Transition Checklist C 01 Rev ADocument4 pagesISO 9001-2015 Transition Checklist C 01 Rev Avikkasverma.in100% (1)
- Quality Management - ISO 9001 - 2015 Mandatory Documented Information - Documents and RecordsDocument6 pagesQuality Management - ISO 9001 - 2015 Mandatory Documented Information - Documents and RecordsSithanandan GanapathyNo ratings yet
- Visio Document DevelopmentDocument1 pageVisio Document DevelopmentManInTheBushNo ratings yet
- UDA Act of Sri LankaDocument25 pagesUDA Act of Sri LankaManInTheBush100% (1)
- FPI Technical CharacteristicsDocument36 pagesFPI Technical Characteristicsfahimshah1301No ratings yet
- Sri Lanka Desk Calendar 2009Document1 pageSri Lanka Desk Calendar 2009ManInTheBushNo ratings yet
- 2006 4 V Foreign Funded Projects EnglishDocument36 pages2006 4 V Foreign Funded Projects EnglishManInTheBush0% (1)
- FLOWTITE Install GuideDocument72 pagesFLOWTITE Install GuideRanjit RjtNo ratings yet
- Sanitary Sewer Design ManualDocument19 pagesSanitary Sewer Design ManualManInTheBushNo ratings yet
- Act No. 13 (E)Document5 pagesAct No. 13 (E)ManInTheBushNo ratings yet
- How To Use The AIS Web Site To Obtain Current NOTAM ListingsDocument10 pagesHow To Use The AIS Web Site To Obtain Current NOTAM ListingsManInTheBushNo ratings yet
- EB - 44 - Coal Tar RejuvenationDocument5 pagesEB - 44 - Coal Tar RejuvenationManInTheBushNo ratings yet
- CFD Jet Engine Wake and Noise Data PDFDocument24 pagesCFD Jet Engine Wake and Noise Data PDFmojicapNo ratings yet
- Case Assignment-Activity Based CostingDocument5 pagesCase Assignment-Activity Based CostingDenny SitumorangNo ratings yet
- 124-Video Conference RFP PDFDocument64 pages124-Video Conference RFP PDFSV BNo ratings yet
- Oriental Engineering Works PVTDocument20 pagesOriental Engineering Works PVTDarshan DhimanNo ratings yet
- Chapter 7Document22 pagesChapter 7anis abdNo ratings yet
- Group 5 - SCM - LL BeanDocument8 pagesGroup 5 - SCM - LL BeanSiddharthNo ratings yet
- Fabm 1Document2 pagesFabm 1hours cityNo ratings yet
- Baryalai Amin: ObjectiveDocument2 pagesBaryalai Amin: Objectivebaryalai aminNo ratings yet
- Toolbox Talk - Traffic ManagementDocument2 pagesToolbox Talk - Traffic ManagementAdrianNo ratings yet
- C6-Intercompany Inventory Transactions PDFDocument43 pagesC6-Intercompany Inventory Transactions PDFVico JulendiNo ratings yet
- Isocertificate PDFDocument1 pageIsocertificate PDFSarmiento HerminioNo ratings yet
- Is-Retail Replenishment Vs Multi-Step ReplenishmentDocument9 pagesIs-Retail Replenishment Vs Multi-Step ReplenishmentNoppawan BuangernNo ratings yet
- The Mobile Store Ltd.Document79 pagesThe Mobile Store Ltd.jamshedansari2020100% (1)
- Law Offices of Harry Finkle, A Professional Corporation Proposal - RedactedDocument5 pagesLaw Offices of Harry Finkle, A Professional Corporation Proposal - RedactedL. A. PatersonNo ratings yet
- Test Bank For Essentials of Corporate Finance 9th Edition by RossDocument24 pagesTest Bank For Essentials of Corporate Finance 9th Edition by Rosspauljohnstonapgsoemyjk98% (45)
- CFI - Accounting - Fundementals - Johannes Period 1 To 3 SolutionDocument5 pagesCFI - Accounting - Fundementals - Johannes Period 1 To 3 SolutionsovalaxNo ratings yet
- Managing GoogleDocument9 pagesManaging GoogleRose Andrea FerreiraNo ratings yet
- Collins Family Shoprite - Social MediaDocument4 pagesCollins Family Shoprite - Social Mediaapi-455406874No ratings yet
- 14 Principles of ManagementDocument21 pages14 Principles of ManagementZoha KhanNo ratings yet
- The Philip Fisher Screen That Fishes Quality StocksDocument3 pagesThe Philip Fisher Screen That Fishes Quality StocksMartinNo ratings yet
- Building & Restructuring The CorporationDocument17 pagesBuilding & Restructuring The CorporationziaeceNo ratings yet
- History of E-CommerceDocument10 pagesHistory of E-CommerceTanushNo ratings yet
- Olx 2Document15 pagesOlx 2sumitNo ratings yet
- R2. TAX (M.L) Solution CMA May-2023 ExamDocument5 pagesR2. TAX (M.L) Solution CMA May-2023 ExamSharif MahmudNo ratings yet
- Anil Iyer 706395148Document4 pagesAnil Iyer 706395148kris_sammetaNo ratings yet
- Worldcom - The Story of A WhistleblowerDocument5 pagesWorldcom - The Story of A WhistleblowerYeni Hanisah PiliangNo ratings yet
- 4QUIZDocument3 pages4QUIZMarilyn Nelmida TamayoNo ratings yet
- Case Study Best Practices at The FedEx CorporationDocument3 pagesCase Study Best Practices at The FedEx CorporationAlpana Varnwal0% (1)
- Arun Sharma CAT (DI and LR) PDFDocument18 pagesArun Sharma CAT (DI and LR) PDFRupasinghNo ratings yet
- To Do Path: How To Define Company StructureDocument55 pagesTo Do Path: How To Define Company Structurepragyaa4No ratings yet
- Advanced Seminar in Business Plan WritingDocument2 pagesAdvanced Seminar in Business Plan Writingshubhendra89No ratings yet