Professional Documents
Culture Documents
117
Uploaded by
faboll_007Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
117
Uploaded by
faboll_007Copyright:
Available Formats
Color profile: Disabled
Composite Default screen
151
Effect of light, salinity, and temperature on seed
germination of Limonium stocksii
Sabahat Zia and M. Ajmal Khan
Abstract: Limonium stocksii (Boiss.) Kuntze (Plumbaginaceae) is a perennial, woody shrub distributed at Hawks Bay,
Karachi, Pakistan. Experiments were carried out to investigate seed germination responses of L. stocksii at different salinities (0, 100, 200, 300, 400, and 500 mmol/L NaCl) and under different temperature regimes (10:20, 15:25, 20:30,
and 25:35 C), both in a 12 h dark : 12 h light photoperiod and in complete darkness. The highest percentage of germination (about 100%) was obtained at 0, 100, and 200 mmol/L NaCl at 20:30 C, and a further increase in salinity
resulted in a gradual decrease in germination. Less than 5% of seeds germinated at 500 mmol/L NaCl. Germination
under salinity treatment at 15:25 C was slightly more inhibitory than the optimal temperature regime, whereas under
both 10:20 and 25:35 C temperature regimes, seed germination was substantially reduced and few seeds germinated at
concentrations higher than 200 mmol/L NaCl. Germination rate was fastest at 20:30 C and slowest at 10:20 C. Relatively low seed germination was obtained in the dark in comparison to seeds germinated in a 12-h photoperiod under
saline conditions. Recovery experiments showed that exposure of seeds to various salinity and temperature regimes had
little effect on viability of seeds.
Key words: germination, light, Limonium stocksii, NaCl, recovery, temperature.
Rsum : Le Limonium stocksii (Boiss.) Kuntze (Plumbaginaceae), est une plante prenne ligneuse arbustive, qui se retrouve Hawks Bay, prs de Karachi, au Pakistan. Les auteurs ont conduit des essais pour tudier le comportement de
la germination des graines du L. stocksii en prsence de diffrentes concentrations de sel (NaCl 0, 100, 200, 300, 400
et 500 mmol/L), sous diffrents rgimes de temprature (10:20, 15:25, 20:30 et 25:35 C), et sous une photopriode de
12 h dobscurit : 12 h de lumire, ainsi quen obscurit totale. On observe la plus forte germination (prs de 100 %)
en prsence de NaCl 0, 100 et 200 mmol/L, et 20:30 C; une augmentation plus pousse de la salinit conduit une
diminution graduelle de la germination, seulement 5 % des graines germant en prsence NaCl 500 mmol/L. La germination en prsence de sel et 15:25 C est lgrement plus faible quau rgime de tempratures optimum, alors qu
10:20 et 25:35 C, la germination des graines est considrablement rduite, seulement quelques graines germant aux
concentrations en NaCl suprieures 200 mmol/L. Les taux de germination sont les plus rapides 20:30 C et les plus
lents 10:20 C. Dans lobscurit, la germination des graines est relativement plus faible comparativement celle
quon obtient avec une photopriode de 12 h, sous des conditions salines. Des essais de rcupration montrent que
lexposition des graines divers rgimes de salinit et de tempratures a peu deffet sur la viabilit des graines.
Mots cls : germination, lumire, Limonium stocksii, NaCl, rcupration, temprature.
[Traduit par la Rdaction]
Zia and Khan
157
Introduction
Halophytes are distributed in coastal and inland saline
habitats throughout the world (Adam 1990; Ungar 1991),
and their populations are subjected to high mortality risks
because of the direct action of high-salinity stress or other
associated abiotic factors (Ungar 1991). Seeds of halophytes
usually show optimal germination in freshwater similar to
glycophytes, but differ in their ability to germinate at higher
salinities (Ungar 1995).
Received 14 April 2003. Published on the NRC Research
Press Web site at http://canjxx.nrc.ca on 27 February 2004.
S. Zia1 and M. Ajmal Khan.2 Department of Botany,
University of Karachi, Karachi 75270, Pakistan.
1
Present address: B.A.M.M., P.E.C.H.S. Government College
for Women, P.E.C.H.S. Society, Karachi.
2
Corresponding author (e-mail: ajmal@halophyte.org).
Can. J. Bot. 82: 151157 (2004)
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:04 AM
Perennial halophytes vary in their ability to tolerate salinity (Khan 2002), and this variation could be due to a number
of factors such as light, temperature, and moisture stress
(Baskin and Baskin 1998; Mahmoud et al. 1983; Noe and
Zedler 2000). Maximum salt tolerance for germination of
subtropical species from the Karachi coast in Pakistan has
been reported for Arthrocnemum macrostachyum (Moric.)
C. Koch (10% germination at 1000 mmol/L NaCl; Khan
and Gul 1998), Cressa cretica L. (3% germination at
1000 mmol/L NaCl; Khan 1999), and Salsola imbricata
Forssk. (6% germination at 800 mmol/L; M.A. Khan, unpublished data). However, a number of species could not
germinate at NaCl concentrations higher than 400 mmol/L
(Khan 2002).
Temperature interacts with salinity to affect the germination of halophyte seeds (Khan et al. 2001). The adverse
effect of high salinity is further aggravated by either an increase or decrease in temperature (Khan and Rizvi 1994;
Khan 2002). Germination of many halophytes occurs at
doi: 10.1139/B03-118
2004 NRC Canada
Color profile: Disabled
Composite Default screen
152
times when there is an optimal combination of day length,
temperature regime, and salinity (Naidoo and Naicker 1992;
Gutterman et al. 1995; Khan 2002). Absence of light almost
completely inhibits seed germination of Triglochin maritima L. (Khan and Ungar 1999) and Sporobolus indicus (L.)
R. Br. (Andrews 1997) and partially inhibits germination in
Apium graveolens L. (Garcia et al. 1995), Allium staticiforme Sibth. & Sm., Brassica tournefortii Gouan, Cakile
maritima Scop., and Onanthus maritimus (L.) Hoffmanns &
Link (Thanos et al. 1991), while seeds of Atriplex stocksii
Boiss. (Khan and Rizvi 1994) and Suaeda fruticosa Forssk.
(Khan and Ungar 1998) are not inhibited by the absence of
light.
Most seeds are located near the soil surface, where salt
concentration changes because of continuous evaporation of
ground water (Ungar 1991). Rainfall can quickly leach salt
from the surface and supply water to the seed. Thus, for successful establishment of plants in saline environments, seeds
must remain viable in high salinity and germinate when salinity decreases (Khan and Ungar 1997). Halophyte seeds
are known to maintain viability for extended periods of time
during exposure to high salinity, and they initiate germination when salinity is reduced (Keiffer and Ungar 1995; Khan
and Ungar 1998, 1999; Khan 2002). Recovery germination
of seeds from hypersaline conditions is affected by the temperature regime to which seeds are exposed (Khan and
Ungar 1997). Halophytic species show a range of responses
from partial to complete germination recovery when salinity
stress is alleviated (Khan 2002).
Limonium stocksii (Boiss.) Kuntze is a low-branched, saltsecreting, woody shrub in the family Plumbaginaceae. It is
distributed in high coastal marshes as well as rocky grounds
near the coast of Pakistan and India (Gujarat). In Karachi,
pure populations of L. stocksii are found at the farthest end
of Manora Creek near Hawks Bay in association with a few
individuals of Arthrocnemum macrostachyum, Aeluropus
lagopoides (L.) Trin. ex. Thw., Urochondra setulosa (Trin.)
C.E. Hubbard, Suaeda fruticosa, Tamarix spp., and Atriplex
stocksii. Possible sources of moisture are the monsoon rains
and oceanic seepage. The monsoon period starts 15 June and
ends 15 September. Owing to high tides during this period,
oceanic seepage increases, while rainfall (220 mm/year) usually occurs during July and August. Storms are rarely reported from the Karachi coast. Limonium stocksii flowers
twice a year beginning in June and November, and a large
number of seeds are produced in August and January. After
dispersal seeds become part of the seed bank and germinate
only after the monsoon rains. The average ambient temperature during the monsoon period ranges from 20 C at night
to 30 C during the day. Limonium stocksii is a highly salttolerant plant that grows in coastal areas under high salinity,
and it is occasionally foraged. The economic potential of
this species as an ornamental plant for coastal saline areas is
great. It is also an important component of littoral ecosystem
of Karachi, Pakistan. Growing this species in its native habitat would preserve its population and reduce grazing pressure.
The aim of the present study was to determine percent
germination, rate of germination, and recovery responses of
L. stocksii under various salinity, temperature, and light conditions.
Can. J. Bot. Vol. 82, 2004
Materials and methods
Seeds of L. stocksii were collected in February 2000, from
a salt flat at the upper end of Manora Creek near Hawks
Bay, Karachi (244525N and 664567E). Seeds were
separated from the inflorescence, surface sterilized using sodium hypochlorite (0.52%) for 1 min, followed by thorough
rinsing with distilled water and air drying. Germination was
carried out using 5 cm diameter, tight-fitting plastic Petri
plates with 5 mL of test solution prepared by using distilled
and deionized water. Each dish was placed in a 10 cm diameter plastic Petri plate as an added precaution against the
loss of water by evaporation. Four replicates of 25 seeds
each were used for each treatment. Seeds were considered to
be germinated at the emergence of the radicle.
To determine the effect of temperature, seeds were germinated in incubators at four alternating temperature regimes
of 10:20, 15:25, 20:30, and 25:35 C. A 24-h cycle was
used, where higher temperatures (20, 25, 30, and 35 C)
coincided with a 12-h light period (Sylvania cool white fluorescent lamps, 25 molm2s1, 400750 nm) and lower
temperatures (10, 15, 20, and 25 C) coincided with a 12-h
dark period. Seeds were germinated at six salinities (0, 100,
200, 300, 400, and 500 mmol/L NaCl) as a result of preliminary tests, which determined the range of salinity tolerance.
Percent germination was recorded on every alternate day for
20 d. Ungerminated seeds were transferred to distilled water
after 20 d to study the recovery of germination, which was
also recorded at 2-d intervals for 20 d.
Seeds were also germinated in complete darkness by placing Petri plates in black plastic bags and then in incubators
at the above-mentioned temperature regimes for 20 d. Percent germination was recorded after 20 d.
Rate of germination was estimated by using a modified
Timsons index of germination velocity, germination velocity = G/t, where G is the percentage of seed germination at
2-d intervals and t is the total germination period (Khan and
Ungar 1997). The maximum value possible for our data using this index was 50 (i.e., 1000/20). The higher the value,
the more rapid the germination. The percent recovery was
determined by the formula (ab)/(cb) 100, where a is the
total number of seeds germinated after being transferred to
distilled water, b is the total number of seeds germinated in
saline solution, and c is the total number of seeds.
Germination data were transformed (arcsine) before a statistical analysis was performed. These data were analyzed
using SPSS Version 6.1 for Windows (SPSS Inc. 1994). A
two-way ANOVA was also used to demonstrate the interaction between various factors in affecting the rate, recovery,
and percent germination. A Bonferroni test was used (P <
0.05) to determine significant differences between means of
percent germination among salinity treatments under various
light and temperature regimes (SPSS Inc. 1994).
Results
A two-way ANOVA indicated significant (P < 0.0001)
individual effects of salinity, temperature, and their interaction on percent germination, rate of germination, and percent
recovery of L. stocksii seeds (Table 1).
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:04 AM
Color profile: Disabled
Composite Default screen
Zia and Khan
153
Fig. 1. Mean final percent germination of Limonium stocksii in various salinity, temperature, and light and dark conditions.Bars having
the same letter within each light treatment are not significantly different (P < 0.05) from the control (Bonferroni test). Bars represent
mean SE.
Table 1. A two-way ANOVA of the effects of salinity (S),
temperature (T), and their interaction on germination of
Limonium stocksii
Dependent variable
ST
% germination
Rate of germination
% recovery
426.7***
668.6***
359.6***
127.7***
229.5***
80.8***
29.2***
42.6***
23.7***
Note: Numbers indicate F values (***, P < 0.001).
Maximum seed germination in light was obtained in nonsaline control under all temperature regimes (Fig. 1). Exposure to different salinity levels resulted in a gradual decrease
in percent germination, and this reduction varied with the
change in temperature regime (Fig. 1). Best germination under saline conditions was observed at 20:30 C treatment,
where germination in 100 and 200 mmol/L NaCl was not
significantly different from the control. A further increase in
salinity decreased germination and only 5% of seeds germinated at 500 mmol/L NaCl. Seed germination at 15:25 C
was comparatively lower than germination under the optimal
temperature regime. Exposure to lower (10:20 C) and
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:04 AM
Color profile: Disabled
Composite Default screen
154
Can. J. Bot. Vol. 82, 2004
Fig. 2. Cumulative mean percent germination of Limonium stocksii seeds over time in 0, 100, 200, 300, 400, and 500 mmol/L NaCl in
12 h light : 12h dark photoperiod. Bars represent mean SE.
higher (25:35 C) temperature regimes substantially inhibited germination in all salinity treatments (Fig. 1), and the
lowest germination was obtained at 10:20 C, where 25%
seed germination was obtained in 100 mmol/L NaCl.
Temperature also affected speed of germination under
both saline and nonsaline conditions (Fig. 2). Maximum germination in the distilled water control was obtained after 2 d
under all temperature regimes except for 10:20 C, where it
was attained in 14 d (Fig. 2). In saline solutions, maximum
germination varied from 6 to 18 d. Under the optimal temperature regime, germination at lower salinity (100 and
200 mmol/L NaCl) peaked in 6 d and it was about 10 d in
higher salinity treatments. However, seed germination peaked at 10 d for all salt concentrations at 15:25 C (Fig. 2).
Seed germination in the distilled water control was not affected by darkness under any temperature regime with the
exception of 10:20 C, where percent germination was reduced to 50% (Fig. 1). Application of salt under complete
darkness greatly reduced percent germination and the level
of inhibition varied with temperature. About 2% of seeds
could germinate in 100 mmol/L NaCl under lower
(10:20 C) and higher (25:35 C) temperatures. Maximum
germination (74%) was obtained at 20:30 C in 100 mmol/L
NaCl, and it decreased to just 8% in 200 mmol/L NaCl. At
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:04 AM
Color profile: Disabled
Composite Default screen
Zia and Khan
15:25 C, 62% of seeds germinated in 100 mmol/L NaCl,
and 16% germination was obtained in 200 mmol/L NaCl
(Fig. 1).
Rate of germination was highest in nonsaline controls except at 10:20 C, and addition of NaCl slowed the rate of
germination (Fig. 3). Temperature also influenced rate of germination. At lower and higher temperatures, seeds showed a
slower rate of germination from 100 to 300 mmol/L NaCl
than at 20:30 C and 15:25 C (Fig. 3).
When light-treated, ungerminated seeds from salt treatments were transferred to distilled water, they recovered completely under all salinity and temperature regimes (Fig. 4).
155
Fig. 3. Rate of germination of Limonium stocksii seeds under
various salinity and thermoperiod treatments. Bars represent
mean SE.
Discussion
Plants native to the subtropical maritime desert of Karachi
are exposed to various levels of moisture and salinity stress
because of an unpredictable monsoon period (Khan 2002).
These conditions lead to different life history strategies in
desert plants that allow the plants to maximize their fitness
(Kigel 1995). Coastal areas of Pakistan are reported to have
around 100 species of halophytes (Khan and Gul 2002), and
salinity tolerance of only a small number of these species is
known. The available reports indicate that dicotyledonous
species vary in their tolerance during germination, including
species such as Arthrocnemum macrostachyum (1000 mmol/L
NaCl; Khan and Gul 1998), Cressa cretica (1000 mmol/L
NaCl; Khan 1999), Salsola imbricata (800 mmol/L NaCl;
M.A. Khan, unpublished data), Suaeda fruticosa
(500 mmol/L NaCl; Khan and Ungar 1998), Atriplex stocksii
(300 mmol/L NaCl; Khan and Rizvi 1994), and some grasses
such as Aeluropus lagopoides (500 mmol/L NaCl; Gulzar and
Khan 2001), Urochondra setulosa (500 mmol/L NaCl; Gulzar
et al. 2001), Halopyrum mucronatum (300 mmol/L NaCl;
Khan and Ungar 2001), and Sporobolus ioclados
(500 mmol/L NaCl; Khan and Gulzar 2003). Limonium stocksii is a moderately salt-tolerant halophyte at germination
when compared with other local halophytic species, but
it has the ability to germinate at salinity levels of up
to 500 mmol/L NaCl, which approach seawater salinity
(600 mmol/L NaCl).
Temperature and salinity interact to affect the germination
of halophytes (Khan and Rizvi 1994; Khan and Ungar 1997,
1998; Khan and Gul 1998). Some species are more sensitive
to changes in temperature (Cressa cretica and Zygophyllum
simplex) than others (Arthrocnemum macrostachyum and
Suaeda fruticosa) (Khan 1999; Khan and Gul 1998; Sheikh
and Mahmood 1986). Seed germination of L. stocksii was
also influenced by temperature. We found 20:30 C to be the
optimal temperature for germination and any increase or decrease in temperature inhibited germination. This inhibition
progressively increased with salinity. Recruitment of L. stocksii in natural conditions through germination appears to
take place after monsoon rains. Germination of halophyte
seeds in subtropical coastal and inland salt marshes usually
occurs after monsoon rains, which causes a reduction in
temperature and lowering of soil salinity (Khan and Gul
1998; Khan and Ungar 1998).
Several reports have indicated that the rate of germination
is more sensitive to salinity than is overall percent germination (West and Taylor 1981; Dudeck and Peacock 1985;
Fig. 4. Mean recovery percent germination for Limonium stocksii
in distilled water under different temperature regimes in various
salinity treatments. Bars represent mean SE.
Marcar 1987). Very rapid germination was reported for Haloxylon recurvum and Haloxylon salicornicum (Sharma and
Sen 1989) and Limonium axillare (Mahmoud et al. 1983),
and they considered it to be a strategy to utilize the brief period of water availability after rainfall. Rogers et al. (1995)
suggested that fast germination ensures rapid seedling establishment, which can minimize competition. Seeds of L. stocksii germinated rapidly in control and in up to 200 mmol/L
NaCl at 20:30 C, a temperature regime similar to the average early summer period in Karachi.
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:05 AM
Color profile: Disabled
Composite Default screen
156
Limonium stocksii seeds displayed a greater tolerance to
high-salinity and temperature stress before germination.
Seeds germinated within 2 d when transferred to nonsaline
media from various salinity treatments and temperature regimes. Similar results were obtained by Mahmoud et al.
(1983) for L. axillare, which showed 95% recovery for
60%100% seawater treatments. Khan and Ungar (1998)
also observed a quick recovery in Suaeda fruticosa seeds at
all temperature regimes. The ability of halophyte seeds to
survive hypersaline conditions and germinate when salinity
is reduced provides them with multiple opportunities for
cohort establishment in unpredictable saline environments
(Khan and Ungar 1997).
Limonium stocksii usually grows in coastal salt marshes
that remain more or less wet during all seasons due to seepage of seawater. Seed reserves in the soil are exposed to
high-temperature stress (around 40 C or higher) while imbibed in seawater during seven months of the year. Seeds
of L. stocksii remain viable under natural conditions after
extended exposure to salinity and temperature stress and germinate readily when salinity and temperature stress are
reduced after monsoon rains (S. Zia and M.A. Khan, unpublished data). Although seeds could only germinate in up to
500 mmol/L NaCl, which is much lower in comparison to
other associated halophytic species, their salinity tolerance
during storage in the seed bank confers a successful reproductive strategy in these unpredictable conditions. Seeds of
species such as Arthrocnemum macrostachyum and Sporobolus ioclados lose viability when exposed to hightemperature and salinity stress (S. Zia and M.A. Khan,
unpublished data). However, Suaeda fruticosa seeds maintain a viability similar to that of L. stocksii. Survival of seeds
under extreme conditions provides this species with a strategy for successful recruitment in a harsh environment. Limonium stocksii is a potential candidate as an ornamental and
fodder crop in coastal areas where only brackish water or
seawater is available.
Acknowledgements
We would like to thank the University of Karachi and the
Higher Education Commission for providing the merit scholarship to S. Zia.
References
Adam, P. 1990. Saltmarsh ecology. Cambridge University Press,
New York.
Andrews, T.S. 1997. Factors affecting the germination of Giant
Parramatta grass. Aust. J. Exp. Agri. 37: 439446.
Baskin, J.M., and Baskin, C.C. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego, Calif., U.S.A.
Dudeck, A.E., and Peacock, C.H. 1985. Salinity effect on perennial
ryegrass germination. Hortscience, 20: 268269.
Garcia, F.P., Pita, J.M., Benito, M., and Iriondo J.M. 1995. Effect
of light, temperature and seed priming on germination of celery
seeds (Apium graveolens L.). Seed Sci. Technol. 23: 377383.
Gulzar, S., and Khan, M.A. 2001. Seed germination of a halophytic
grass Aeluropus lagopoides. Ann. Bot. (Lond.), 87: 319324.
Can. J. Bot. Vol. 82, 2004
Gulzar, S., Khan, M.A., and Ungar, I.A. 2001. Effect of salinity
and temperature on the germination of Urochondra setulosa
(Trin.) C.E. Hubbard. Seed Sci. Technol. 29: 2129.
Gutterman, Y., Kamenetsky, R., and Van Rooyen, M. 1995. A comparative study of seed germination of two Allium species from
different habitats in the Negev desert highlands. J. Arid Environ.
29: 305315.
Keiffer, C.W., and Ungar, I.A. 1995. Germination responses of
halophyte seeds exposed to prolonged hypersaline conditions. In
Biology of salt tolerant plants. Edited by M.A. Khan and I.A.
Ungar. Department of Botany, University of Karachi, Pakistan.
pp. 4350.
Khan, M.A. 1999. Comparative influence of salinity and temperature on the germination of subtropical halophytes. In Halophyte
uses in different climates. I: ecological and ecophysiological
studies. Vol. 13. Edited by H. Lieth, M. Moschenko, M. Lohman, H.W. Koyro, and A. Hamdy. Backhuys Publishers, Leiden.
pp. 7788.
Khan, M.A. 2002. Halophyte seed germination: success and pitfalls. In Proceedings of the International Symposium on Optimum Resources Utilization in Salt-Affected Ecosystems in Arid
and Semi-arid Regions, Cairo, 811 April 2002. Edited by A.M.
Hegazi, H.M. El-Shaer, S. El-Demerdashe, R.A. Guirgis, A.A.
Metwally, F.A. Hassan, and H.E. Khashaba. Desert Research Institute, Cairo, Egypt. pp. 346358.
Khan, M.A., and Gul, B. 1998. High salt tolerance in germinating
dimorphic seeds of Arthrocnemum indicum. Int. J. Plant Sci.
159: 826832.
Khan, M.A., and Gul, B. 2002. Salt tolerant plants of coastal sabkhas of Pakistan. In Sabkha ecosystems. Edited by H. Barth and
B. Boer. Kluwer Academic Press, Netherlands. pp. 123129.
Khan, M.A., and Gulzar, S. 2003. Germination responses of Sporobolus ioclados: a potential forage grass. J. Arid Environ. 53:
387394.
Khan, M.A., and Rizvi, Y.1994. Effect of salinity, temperature, and
growth regulators on the germination and early seedling growth
of Atriplex griffithii var. stocksii. Can. J. Bot. 72: 475479.
Khan, M.A., and Ungar, I.A. 1997. Effect of thermoperiod on recovery of seed germination of halophytes from saline conditions. Am. J. Bot. 84: 279283.
Khan, M.A., and Ungar, I.A. 1998. Germinaion of salt tolerant
shrub Suaeda fruticosa from Pakistan: salinity and temperature
responses. Seed Sci. Technol. 26: 657667.
Khan, M.A., and Ungar, I.A. 1999. Seed germination and recovery
of Triglochin maritima from salt stress under different thermoperiods. Great Basin Nat. 59: 144150.
Khan, M.A., and Ungar, I.A. 2001. Alleviation of salinity stress
and the response to temperature in two seed morphs of Halopyrum mucronatum (Poaceae). Aust. J. Bot. 49: 777783.
Khan, M.A., Gul, B., and Weber, D.J. 2001. Effect of salinity and
temperature on the germination of Kochia scoparia. Wetlands
Ecol. and Manage. 9: 483489.
Kigel, J. 1995. Seed germination in arid and semi-arid regions. In
Seed development and germination. Edited by J. Kigel and G.
Galili. Marcel Dekker, New York. pp. 645700.
Mahmoud, A., El Sheikh, A.M., and Abdul Baset, S. 1983. Germination of two halophytes: Halopeplis perfoliata and Limonium
axillare from Saudi Arabia. J. Arid Environ. 6: 8798.
Marcar, N. 1987. Salt tolerance in the genus Lolium (ryegrass) during germination and growth. Aust. J. Agri. Res. 38 :297307.
Naidoo, G., and Naicker, K. 1992. Seed germination in the coastal
halophytes Triglochin bulbosa and Triglochin striata. Aquat.
Bot. 42: 217229.
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:05 AM
Color profile: Disabled
Composite Default screen
Zia and Khan
Noe, G.B., and Zedler, J.B. 2000. Differential effects of four abiotic factors on the germination of salt marsh annuals. Am. J. Bot.
87: 16791692.
Rogers, M.E., Noble, C.L., Halloran, G.M., and Nicolas, M.E.
1995. The effect of NaCl on germination and early seedling
growth of white clover (Trifolium repens L.) population selected
for high and low salinity tolerance. Seed Sci. Technol. 23: 277
287.
Sharma, T.P., and Sen, D.N. 1989. A new report on abnormally fast
germinating seeds of Haloxylon spp.: an ecological adaptation to
saline habitat. Current Sci. 58: 382385.
Sheikh, K.H., and Mahmood, K. 1986. Some studies on field distribution and seed germination of Suaeda fruticosa and Sporobolus arabicus with reference to salinity and sodicity of the
medium. Plant Soil, 94: 333340.
157
SPSS Inc. 1994. SPSS Version 6.1 [computer program]. SPSS Inc.,
Chicago, Ill.
Thanos, C.A., Georghiou, K., Douma, D.J., and Marangaki, C.J.
1991. Photoinhibition of seed germination in Mediterranean
maritime plants. Ann. Bot. (Lond.), 68: 469475.
Ungar, I.A. 1991. Ecophysiology of vascular halophytes. CRC
Press, Boca Raton, U.S.A.
Ungar, I.A. 1995. Seed germination and seed-bank ecology in
halophytes. In Seed development and germination. Edited by J.
Kigel and G. Galili. Marcel Dekker, New York. pp. 529544.
West, D.W., and Taylor, J.A. 1981. Germination and growth of
cultivars of Trifolium subterraneum L. in the presence of sodium
chloride salinity. Plant Soil, 62: 221230.
2004 NRC Canada
J:\cjb\cjb8202\B03-118.vp
February 23, 2004 8:10:05 AM
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Diagnosis Cure Treatment, Disease Prevention: Sources of Drug InformationDocument11 pagesDiagnosis Cure Treatment, Disease Prevention: Sources of Drug InformationShyen100% (11)
- Daniel Pimley - Representations of The Body in AlienDocument37 pagesDaniel Pimley - Representations of The Body in AlienVerbal_KintNo ratings yet
- Chapter 2 W PhotosDocument37 pagesChapter 2 W PhotosIndra ChristianNo ratings yet
- Twincubator Use and Maintenance: Document Type: SOPDocument4 pagesTwincubator Use and Maintenance: Document Type: SOPfelix bazanNo ratings yet
- Conduction of DeliveryDocument31 pagesConduction of Deliverysagi mu100% (2)
- AMICON Ultra Centrifuge DevicesDocument9 pagesAMICON Ultra Centrifuge Devicesdjass1804No ratings yet
- Solutions, Suspension and Colloidal SystemDocument27 pagesSolutions, Suspension and Colloidal SystemmrvenkateshNo ratings yet
- D7 11 June Paper 2015finDocument12 pagesD7 11 June Paper 2015finnoxolo dwaneNo ratings yet
- Origin of Life 1Document2 pagesOrigin of Life 1zxcvblesterNo ratings yet
- Ex. 5 DNA Extraction PDFDocument3 pagesEx. 5 DNA Extraction PDFAlyssa Pauline PalacioNo ratings yet
- 4HB0 Revision NotesDocument4 pages4HB0 Revision NotesTamer A. DakkakNo ratings yet
- Restriction Enzymes: Restriction Enzymes Is An Enzyme That Cuts Double Stranded or SingleDocument3 pagesRestriction Enzymes: Restriction Enzymes Is An Enzyme That Cuts Double Stranded or SingleArafat MiahNo ratings yet
- 3 Carbohydrates' StructureDocument33 pages3 Carbohydrates' StructureDilan TeodoroNo ratings yet
- SCI 11 Module 4Document10 pagesSCI 11 Module 4Sebastian SmytheNo ratings yet
- Preparation and Evaluation of Eye-Drops For The Treatment of Bacterial ConjunctivitisDocument8 pagesPreparation and Evaluation of Eye-Drops For The Treatment of Bacterial ConjunctivitisNur HudaNo ratings yet
- Biology - Lab 5 - Collection and Preservation of AnimalsDocument6 pagesBiology - Lab 5 - Collection and Preservation of AnimalsBeyonce SkekelNo ratings yet
- Valucare: Plan Coordinator Room# ScheduleDocument2 pagesValucare: Plan Coordinator Room# ScheduleAnonymous HH3c17osNo ratings yet
- Mader/Biology, 11/e - Chapter OutlineDocument5 pagesMader/Biology, 11/e - Chapter Outlineapi-455371000No ratings yet
- Bact Growth CurveDocument12 pagesBact Growth Curvehitesh100% (1)
- Metamorphosis Rock, Paper, Scissors: Teacher Lesson PlanDocument6 pagesMetamorphosis Rock, Paper, Scissors: Teacher Lesson Planriefta89No ratings yet
- Nutritional and Antinutritional Composition of Fermented Foods Developed by Using Dehydrated Curry LeavesDocument8 pagesNutritional and Antinutritional Composition of Fermented Foods Developed by Using Dehydrated Curry LeavesManu BhatiaNo ratings yet
- 7 Endemic Centers - MathodologyDocument21 pages7 Endemic Centers - MathodologyMirza ČelebičićNo ratings yet
- Blood Film Preparation and Staining ProceduresDocument14 pagesBlood Film Preparation and Staining ProceduresGedefaw TigabuNo ratings yet
- BIOC7001 - Advanced DNA Techniques 2011 - FINALDocument92 pagesBIOC7001 - Advanced DNA Techniques 2011 - FINALMao NanNo ratings yet
- Chapter 24 HWDocument5 pagesChapter 24 HWFola SolarinNo ratings yet
- Reading (In-Class) 5Document6 pagesReading (In-Class) 5Trân TúNo ratings yet
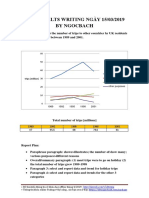
- Giai de Ielts Writing Ngay 150319 by NgocbachDocument7 pagesGiai de Ielts Writing Ngay 150319 by NgocbachSedeaNo ratings yet
- Potential Application of Bacteria To Improve The Strength of Cement ConcreteDocument4 pagesPotential Application of Bacteria To Improve The Strength of Cement ConcreteTaryNo ratings yet
- 5 Enzymes: 0610 BiologyDocument9 pages5 Enzymes: 0610 BiologyAbdul HadiNo ratings yet
- Morph Overfiew - Docx Versi 1Document27 pagesMorph Overfiew - Docx Versi 1Bimo PamungkasNo ratings yet