Professional Documents
Culture Documents
Atlas of Vesicovaginal Fistula
Uploaded by
Yodi SoebadiCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atlas of Vesicovaginal Fistula
Uploaded by
Yodi SoebadiCopyright:
Available Formats
1063-5777/00 $15.00 + .
OO
VAGINAL RECONSTRUCTIVE SURGERY
Vesicovaginal Fistula
Craig V. Comiter, MD, Sandip P. Vasavada, MD, and Shlomo Raz, MD
In developing countries, ischemic injury secondary to obstructed labor is the
leading cause of vesicovaginal fisutla (VVF). In the United States, most VVFs result
from iatrogenic injury. Trauma during gynecologic surgery is responsible for 90% of
WFs, with transabdominal hysterectomy accounting for most cases. Other less
common causes include radiation-induced injury and locally advanced neoplasms
( e g , cervical, endometrial, and vaginal carcinomas).
INDICATIONS FOR SURGERY
Ten percent of small iatrogenic VVFs will close spontaneously with continuous
bladder drainage and antibiotics. If the fistula has not closed after 3 weeks of
catheter drainage, it is unlikely that the fistula will close without surgical intervention. When the fistula is extremely small (1 mm), coagulation of the fistulous tract
may occasionally be successful. Immediate surgical repair is indicated when the
fistula is large enough that most of the urine passes per vagina in spite of continuous bladder drainage. Estrogen replacement is begun at the time of diagnosis in
hypoestrogenic women, and continued until the time of surgery. With an otherwise
healthy patient, success rates have not been shown to differ with early versus
delayed W F repair.
CONTRAINDICATIONS TO SURGERY
Early repair is not recommended in patients with untreated vaginal cuff or
pelvic infection. In such instances, prolonged antibiotic therapy is necessary prior to
repair and reconstruction. Furthermore, operative repair in ill patients is delayed
until the health status improves enough to tolerate surgery. Additionally, surgery
should not be performed in cases of ischemic fistula until the area of necrosis
stabilizes. The transvaginal route is contraindicated in patients who cannot tolerate
the lithotomy position, or in those with severe vaginal stenosis. In cases of a small
capacity bladder (secondary to radiation) in which a concomitant augmentation
cystoplasty is planned, a transabdominal repair of the fistula is indicated.
From the Department of Urology, University of Arizona, Tucson, Arizona (CVC), the Department of
Urology, Thomas Jefferson University, Philadelphia, Pennsylvania (SP), and the Department of Urology, University of California, Los Angeles, California (RS)
ATLAS OF THE UROLOGIC CLINICS OF NORTH AMERICA Volume 8 Number 1 April 2000
133
134
COMITER et al
DIAGNOSIS
Patients typically present with continuous daytime and nighttime leakage per
vagina, with a recent history of gynecologic surgery. Depending on the size of the
fistula, and thereby the ability to store urine in the bladder, the amount of urine
voided versus the amount lost per vagina will vary. Most causes of W F resulting
from surgical trauma are clinically apparent within 10 days of surgery. On the other
hand, radiation-induced VVF may not present until 20 years after radiotherapy.
Pelvic examination often identifies the fistulous opening in the vagina. If the
examination is unrevealing, and the suspicion remains high, the bladder may be
catheterized and filled with a colored solution. The vagina may then be inspected
for leakage. Additionally, the vagina may be packed with a tampon, and the vagina
re-examined after ambulation. If suspicion still remains high, intravenous indigo
carmine or oral phenazopyridine may help to diagnose a uretero-vaginal fistula.
In any patient with a suspected or confirmed fistula, voiding cystourethrography, cystoscopy, and upper tract evaluation are indicated. Voiding cystourethrography may demonstrate the fistula and any concomitant prolapse. Cystoscopy is
necessary to evaluate bladder capacity, the size and location of the fistula, and its
relation to the ureteral orifices. Biopsy is recommended if there is a history of
genitourinary malignancy. Upper tract evaluation is useful to rule out ureterovaginal fistula or ureteral obstruction.
VAGINAL REPAIR OF SIMPLE VVF
The patient should be in the dorsal lithotomy position. A rectal pack helps the
surgeon to identify the rectum, especially if a peritoneal flap is incorporated into the
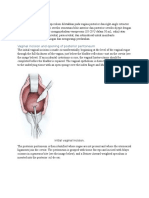
repair (See Figs. 1 to 5).
Figure 1. The fistula is dilated with sounds and an 8F Foley catheter is inserted through the tract. The catheter balloon is filled with 1
to 2 mL of water, and the catheter is used for traction. The vaginal
wall is filled with saline to aid the subsequent dissection. The fistula
is circumscribed and the incision is extended as an inverted J, with
the long arm of the J ending at the vaginal apex. (From Raz S:
Atlas of Transvaginal Surgery. Philadelphia, WB Saunders, 1992, p
147.)
VESICOVAGINAL FISTULA
Figure 2. Vaginal wall flaps are created by dissecting the
vaginal wall for 2 to 4 cm away from the incision proximally, distally, and laterally. The perivesical fascia is exposed, and the circumscribed fistula opening is left intact.
Excision of the tract risks enlarging the communication
between the bladder and vagina, as well as bleeding from
the edges of the fistula. By leaving the fistula in situ, the
vaginal flaps are created in healthy tissue, minimizing
bleeding and permitting a tension-free closure of the defect.
Figure 3. After removing the intrafistula catheter, the fistula
tract is closed transversely with interrupted 2-0 SAS. Sutures
are placed 2 to 3 mm from the edge of the fistula (in healthy
tissue), and incorporate the bladder wall and the epithelialized
tract.
135
136
COMITER et al
Figure 4. The second layer is closed in an imbricating fashion
with 2-0 SAS. This second layer lies perpendicular to the first
layer, thereby minimizing suture overlap. The sutures should enter the perivesical fascia and detrusor muscle 5 rnm from the
previous line of closure. This second layer inverts the prior line
of closure. The bladder should be filled with methylene blue or
indigo carmine solution to evaluate the integrity of the repair. In
cases of a high vesicovaginal fistula, we will harvest a peritoneal
flap to be placed over the first two layers of repair (described
below, see Fig. 7).
Figure 5. The distal vaginal flap is excised and the
proximal flap is advanced anteriorly 3 cm beyond the
fistula repair. This third layer is closed with runninglocking 2-0 SAS, covering the site with healthy vaginal
tissue, while avoiding suture line overlap.
VESICOVAGINAL FISTULA
POSTOPERATIVE CARE
The vagina is packed with antibiotic-impregnated gauze, which may be removed after several hours. The suprapubic and urethral catheters are joined to a Yconnector, and left in place for 10 days. An oral cephalosporin or fluoroquinolone is
continued until the catheters are discontinued, and cholinolytics are given to minimize bladder spasms. Before catheter removal, a voiding cystogram is performed to
evaluate the integrity of the repair. Sexual relations may resume after 12 weeks.
EARLY COMPLICATIONS
Early complications include vaginal bleeding, bladder spasms, and urinary or
vaginal infection. Intraoperative bleeding should be controlled with suture ligation,
minimizing electrocautery. Postoperative bleeding is usually controlled by vaginal
packing and bed rest. Bladder spasms can be treated with cholinolytics. Vaginal or
urinary infection should be treated with oral antibiotics.
LATE COMPLICATIONS
Late complications include vaginal stenosis and foreshortening, unrecognized
ureteral injury, and fistula recurrence. Vaginal shortening or stenosis usually results
from overaggressive resection of vaginal tissue during construction of the vaginal
wall flaps. Delayed recognition of a ureteral injury is best managed by percutaneous
drainage, with definitive surgical repair only after several months, so as not to
jeopardize the fistula repair. Recurrent fistula mandates reoperation. The second
repair is most efficacious when delayed for several months, until the inflammation
associated with the original surgery has completely subsided. Martius flap or peritoneal flap interposition is recommended for repair of recurrent vesicovaginal fistula.
HIGH-RISK FISTULAE
In cases of recurrent fistulae, radiation-induced fistulae, or ischemic (obstetric)
fistulae, and when the fistula is high in the vaginal vault or associated with poor
tissue quality (hypoestrogenic states), the interposition of another source of healthy
tissue is recommended. The most commonly interposed tissues are the Martius
fibrofatty labial graft and the peritoneal flap.
137
138
COMITER et al
MARTIUS GRAFT
See Figure 6.
Figure 6. Martius graft. A vertical incision is made in the labia
majora. Borders of dissection include the labiocrural fold laterally,
Colles fascia covering the urogenital diaphragm posteriorly, and
the bulbocavernosus muscle and labia minora medially. The
blood supply may be based inferiorly on the inferior labial artery,
or superiorly on the external pudendal artery. During mobilization
of the graft, the lateral blood supply (via the obturator artery) is
sacrificed, and either the superior or inferior blood supply must
be severed. A tunnel is created between the perivaginal tissue
and the vaginal wall at the site of the fistula repair, and the
fibrofatty flap is transferred to cover the fistula. The graft is then
secured in place with interrupted 2-0 SAS. The vaginal wall flap
is advanced over the Martius graft, and closed with runninglocking 2-0 SAS as described above. When the vagina is deep,
a Martius flap may not easily reach the area of repair without a
difficult and extensive dissection. The authors reserve the labial
fibrofatty flap for distal fistulae (trigone, bladder neck, or urethra).
VESICOVAGINAL FISTULA
PERITONEAL FLAP
See Figure 7
Figure 7. Peritoneal flap. Unlike the Martius graft, constructing a peritoneal flap does not require extra-vaginal
harvesting. After raising the vaginal wall flaps, the posterior flap is further dissected to the cul-de-sac. The preperitoneal fat and peritoneum are sharply mobilized in a caudal direction. After closing the initial two layers of the
fistula, the peritoneal flap is advanced over the suture line
and sewn in place with interrupted SAS. Finally the vaginal flap is placed over the peritoneal flap, and closed with
running-locking 2-0 SAS. Over the past several years the
authors have been using a peritoneal flap in all vesicovaginal fistula repairs, owing to the simplicity of the technique.
RESULTS
Using the aforementioned technique, with a median follow-up of 5 years (range
6 months-12 years), cure rate was 93% at our institution. Of note, 60% of the
women had failed a previous W F repair.
Address reprint requests to
Shlomo Raz, MD
Professor of Urology
924 Westwood Blvd
Suite 520
Los Angeles, CA 90024
e-mail:sraz@ucla.edu
139
You might also like
- KetamineDocument27 pagesKetaminesamfarmer3330% (2)
- Cardiac Catheterization and Coronary Intervention (2nd Edition, 2020)Document353 pagesCardiac Catheterization and Coronary Intervention (2nd Edition, 2020)John Cancel50% (2)
- 2 GYNE 1a - Benign Lesions of The Vagina, Cervix and UterusDocument11 pages2 GYNE 1a - Benign Lesions of The Vagina, Cervix and UterusIrene FranzNo ratings yet
- 2 2017 03 26!08 03 00 PMDocument10 pages2 2017 03 26!08 03 00 PManakayamNo ratings yet
- Medication Order ReviewDocument34 pagesMedication Order ReviewVidho RiveraNo ratings yet
- Mullarian AnomoliesDocument85 pagesMullarian AnomoliesPrathibha GuruguriNo ratings yet
- (Explorations in Mental Health) Diana J. Semmelhack, Larry Ende, Arthur Freeman, Clive Hazell, Colleen L. Barron, Garry L. Treft-The Interactive World of Severe Mental Illness_ Case Studies of the U.SDocument255 pages(Explorations in Mental Health) Diana J. Semmelhack, Larry Ende, Arthur Freeman, Clive Hazell, Colleen L. Barron, Garry L. Treft-The Interactive World of Severe Mental Illness_ Case Studies of the U.SRey Jerly Duran BenitoNo ratings yet
- Vaginal HysterectomyDocument7 pagesVaginal HysterectomySleepy Winter0% (1)
- Vesicovaginal Fistula: Urology DepartmentDocument24 pagesVesicovaginal Fistula: Urology DepartmentHardiTariqHamma100% (1)
- Abdominoperineal Resection MilesDocument17 pagesAbdominoperineal Resection MilesHugoNo ratings yet
- Hematology Cell Morphology ChartDocument2 pagesHematology Cell Morphology ChartMiaoNo ratings yet
- AnastomosisDocument7 pagesAnastomosissuri84No ratings yet
- 5.thyroid Gland LectureDocument96 pages5.thyroid Gland Lecturesdsher100% (3)
- Salpingo OophorectomyDocument2 pagesSalpingo OophorectomyKasamapon Oak ChawanachitNo ratings yet
- Uretrostomia AtlasDocument10 pagesUretrostomia AtlasquirinalNo ratings yet
- Anorectal Abscess: Principles of Internal Medicine, 18E. New York, Ny: Mcgraw-Hill 2012Document7 pagesAnorectal Abscess: Principles of Internal Medicine, 18E. New York, Ny: Mcgraw-Hill 2012Irene SohNo ratings yet
- Advanced Practice RolesDocument16 pagesAdvanced Practice RolesLorelie Asis0% (1)
- NCLEX Practice ExamDocument10 pagesNCLEX Practice ExamJune DumdumayaNo ratings yet
- Why Stomach Acid Is Essential For Our HealthDocument6 pagesWhy Stomach Acid Is Essential For Our HealthGabriel Turlac100% (1)
- 2017 @dentallib J Jyotsna Rao Quick Review Series For BDS, 4th YearDocument1,066 pages2017 @dentallib J Jyotsna Rao Quick Review Series For BDS, 4th YearYaser Jas25% (4)
- Appendicectomy Step by Step PDFDocument9 pagesAppendicectomy Step by Step PDFOlugbenga A Adetunji100% (1)
- NGT LavageDocument16 pagesNGT LavageTina Alteran100% (1)
- Surgical Mangement VVF BhuvneshwarDocument5 pagesSurgical Mangement VVF BhuvneshwarMangesh NarwadkarNo ratings yet
- Rectovaginal Fistula RepairDocument3 pagesRectovaginal Fistula Repairnaftalina7No ratings yet
- Chapter 132 Total Penectomy. Hinman. 4th Ed. 2017Document3 pagesChapter 132 Total Penectomy. Hinman. 4th Ed. 2017Urologi JuliNo ratings yet
- Umbilicalherniarepair TechniqueDocument9 pagesUmbilicalherniarepair Techniqueanz_4191No ratings yet
- Total Pelvic ExenterationDocument13 pagesTotal Pelvic ExenterationRirin Wahyuni100% (1)
- RRM's Next - Urology-Penis & UrethraDocument32 pagesRRM's Next - Urology-Penis & UrethrairfanNo ratings yet
- Williams2013 Article LaparoscopicManagementOfHighTrDocument3 pagesWilliams2013 Article LaparoscopicManagementOfHighTrVishnu priya kokkulaNo ratings yet
- Urinary DiversionDocument11 pagesUrinary Diversionvlad910No ratings yet
- Pancreaticoj EjunostomyDocument9 pagesPancreaticoj EjunostomyBogdan TrandafirNo ratings yet
- Dr. Pranaya Kumar Panigrahi: PG StudentDocument104 pagesDr. Pranaya Kumar Panigrahi: PG Studentprabowoaji12No ratings yet
- Rectovaginal and Anovaginal Fistulas - UpToDateDocument39 pagesRectovaginal and Anovaginal Fistulas - UpToDateROzi BarriosNo ratings yet
- Surgery of The MareDocument14 pagesSurgery of The MareVictor AvilaNo ratings yet
- VVF RepairDocument4 pagesVVF RepairAdil KhurshidNo ratings yet
- Sotelo Et Al, Laparoscopic Rectovesical Fistula RepairDocument5 pagesSotelo Et Al, Laparoscopic Rectovesical Fistula RepairjordynixnNo ratings yet
- Vesico-Vaginal FistulaDocument8 pagesVesico-Vaginal FistulaBinita ShakyaNo ratings yet
- Placenta Percreta and UrologistDocument4 pagesPlacenta Percreta and Urologistcb_cristianNo ratings yet
- Dr. Trika-Pit Hugi TIDocument37 pagesDr. Trika-Pit Hugi TIMunifah YusriyahNo ratings yet
- Modified Manchester de BoerDocument7 pagesModified Manchester de BoerGustavo flores quispeNo ratings yet
- Pancreaticogastrostomy: Gerard V. Aranha, MD, FRCSC, FACSDocument7 pagesPancreaticogastrostomy: Gerard V. Aranha, MD, FRCSC, FACSBogdan TrandafirNo ratings yet
- Dismembered PyeloplastyDocument7 pagesDismembered PyeloplastymichaelNo ratings yet
- 1 s2.0 S0090429520301618 MainDocument6 pages1 s2.0 S0090429520301618 Mainrivai anwarNo ratings yet
- Abdominoperineal ResectionDocument17 pagesAbdominoperineal ResectionOhana S.No ratings yet
- Atresia Esofágica Con Fistula Traqueoesofágica DistalDocument24 pagesAtresia Esofágica Con Fistula Traqueoesofágica DistalChristian PA100% (1)
- Trauma Urethra: Khenza Nur HasanahDocument32 pagesTrauma Urethra: Khenza Nur HasanahkensaNo ratings yet
- The Spleen: Splenic Trauma and Splenectomy: TrunkDocument3 pagesThe Spleen: Splenic Trauma and Splenectomy: TrunkAsish GeiorgeNo ratings yet
- Vesico Vaginal FistulaDocument6 pagesVesico Vaginal Fistulaapi-3705046No ratings yet
- Abdominal Approach To Vesicovaginal FistulaDocument12 pagesAbdominal Approach To Vesicovaginal FistulaPoto SucioNo ratings yet
- Cloacal MalformationsDocument6 pagesCloacal MalformationsRayhanun MardhatillahNo ratings yet
- Surgical Management of Biliary Diseases Jamie R. Bellah, DVM, Diplomate ACVSDocument2 pagesSurgical Management of Biliary Diseases Jamie R. Bellah, DVM, Diplomate ACVSArokiya Anand KumarNo ratings yet
- Ovariectomia Vs OvariohisterectomiaDocument5 pagesOvariectomia Vs OvariohisterectomiaRicardo CaveroNo ratings yet
- Materi Rupture BladderDocument4 pagesMateri Rupture Bladderendah desfindaNo ratings yet
- Metro Fan ofDocument3 pagesMetro Fan ofsahandNo ratings yet
- VaricocelectomyDocument10 pagesVaricocelectomyRj TanNo ratings yet
- EsophagusDocument168 pagesEsophagusnancy voraNo ratings yet
- Articulo 1 Quiste ColeDocument4 pagesArticulo 1 Quiste ColeMaria Elena VinuezaNo ratings yet
- ISMT 12 - Day 384 - Bob - Lumboperitneal ShuntingDocument11 pagesISMT 12 - Day 384 - Bob - Lumboperitneal ShuntingVito MasagusNo ratings yet
- Management of Anal FistulaDocument5 pagesManagement of Anal Fistulailham adhaniNo ratings yet
- Management of Bulbar Urethral StricturesDocument14 pagesManagement of Bulbar Urethral Stricturesnathaniel ManumbuNo ratings yet
- Genital FistulaeDocument15 pagesGenital Fistulaesangeetha francisNo ratings yet
- Oesophagocoloplasty For Corrosive Oesophageal Stricture: AbstractDocument12 pagesOesophagocoloplasty For Corrosive Oesophageal Stricture: AbstractSpandan KadamNo ratings yet
- Abdominal Hysterectomy TechniqueDocument8 pagesAbdominal Hysterectomy TechniqueDavid Oktavianus TambunanNo ratings yet
- SALPINGECTOMY SALPINGOTOMY ProceduerDocument22 pagesSALPINGECTOMY SALPINGOTOMY ProceduerMuthiah nurul IzzahNo ratings yet
- Female ReproductiveDocument13 pagesFemale Reproductiveta CNo ratings yet
- Total Abdominal HysterectomyDocument19 pagesTotal Abdominal HysterectomyMeidina Rachma Amanda100% (1)
- Cirugías de La Glándula Mamaria en El BovinoDocument20 pagesCirugías de La Glándula Mamaria en El BovinoJavier HernandezNo ratings yet
- CHAPTER 53 OrchidopexyDocument4 pagesCHAPTER 53 OrchidopexyDalia OrellanaNo ratings yet
- Laparoscopic Common Bile Duct ExplorationDocument2 pagesLaparoscopic Common Bile Duct ExplorationmhmmdfaizalNo ratings yet
- Suprapubic Cystostomy: PercutaneousDocument6 pagesSuprapubic Cystostomy: PercutaneousDream ProjectNo ratings yet
- KDI306 - L34 Tramed BOF - IVUDocument93 pagesKDI306 - L34 Tramed BOF - IVUYodi SoebadiNo ratings yet
- Digital Video Recorder Hilux 1Document26 pagesDigital Video Recorder Hilux 1Yodi SoebadiNo ratings yet
- Patent US6447462Document15 pagesPatent US6447462Yodi SoebadiNo ratings yet
- Running AdvancedDocument1 pageRunning AdvancedYodi SoebadiNo ratings yet
- NSAMR Statistics Guide PDFDocument11 pagesNSAMR Statistics Guide PDFYodi SoebadiNo ratings yet
- Guide For Authors: Aims and ScopeDocument11 pagesGuide For Authors: Aims and ScopeYodi SoebadiNo ratings yet
- ClindamycinDocument3 pagesClindamycinWinter KimNo ratings yet
- Clinical Usefulness of Propofol As An Anesthetic Induction Agent in Dogs and CatsDocument3 pagesClinical Usefulness of Propofol As An Anesthetic Induction Agent in Dogs and CatsSatria Adi MarhendraNo ratings yet
- Gemsical Cap (Menrik Biomerge PVT LTD)Document3 pagesGemsical Cap (Menrik Biomerge PVT LTD)Nani GangNo ratings yet
- Harjïa, H. C. L. (0 1 ) - Sfjdldhïa Hckcral (:a. CM.) - Japïtuld:, Páhfka : A 8 . Oæxfjd, M.E., OR7 Lardussc - Hrupd Cmftdrfal Atrfa. Pcjupcramd MCDocument7 pagesHarjïa, H. C. L. (0 1 ) - Sfjdldhïa Hckcral (:a. CM.) - Japïtuld:, Páhfka : A 8 . Oæxfjd, M.E., OR7 Lardussc - Hrupd Cmftdrfal Atrfa. Pcjupcramd MCSofia PerezNo ratings yet
- Thyroid Gland (Mam Suba)Document2 pagesThyroid Gland (Mam Suba)Mark Vincent SahagunNo ratings yet
- Hepatic Encephalopathy in Adults - Treatment - UpToDateDocument8 pagesHepatic Encephalopathy in Adults - Treatment - UpToDatenaka_thanatosNo ratings yet
- Knee ReplacementnhschoicesDocument4 pagesKnee ReplacementnhschoicesCotard DelusionNo ratings yet
- Okc Vs CKC in PfpsDocument10 pagesOkc Vs CKC in PfpsABDUL NASAR.MNo ratings yet
- Case Study: Dengue Hemorrhagic FeverDocument8 pagesCase Study: Dengue Hemorrhagic FeverNichael John Tamorite RoblonNo ratings yet
- 3rd Year Syllabus-202Document24 pages3rd Year Syllabus-202Sahal ShaikhNo ratings yet
- Red Tide: Causative AgentDocument2 pagesRed Tide: Causative Agentchristian quiaoitNo ratings yet
- Journal of Interventional Medicine: Yanli Wang, Guohao Huang, Tian Jiang, Xinwei HanDocument5 pagesJournal of Interventional Medicine: Yanli Wang, Guohao Huang, Tian Jiang, Xinwei HanDzulRizkaNo ratings yet
- Glossopharyngeal Nerve Injury Following Tonsillectomy 5542Document4 pagesGlossopharyngeal Nerve Injury Following Tonsillectomy 5542Kenza SeddikNo ratings yet
- Impaired Gas ExchangeDocument1 pageImpaired Gas Exchangeruggero07No ratings yet
- ESR Microsed PermaiDocument20 pagesESR Microsed PermaiHishamudin RaisNo ratings yet
- The Cardiovascular SystemDocument43 pagesThe Cardiovascular Systemtravis efraimNo ratings yet
- Respiratory SystemDocument7 pagesRespiratory SystemNgọc Mai Khanh NguyễnNo ratings yet
- Apicoectomy - An Overview of Endodontic SurgeryDocument8 pagesApicoectomy - An Overview of Endodontic Surgeryhari_dhbNo ratings yet
- Couvade Syndrome and PseudocyesisDocument4 pagesCouvade Syndrome and PseudocyesisJustin Ahorro-DionisioNo ratings yet