Professional Documents
Culture Documents
Alcohols and Modern Analytical Techniques HW Ms
Uploaded by
Raiyan RahmanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alcohols and Modern Analytical Techniques HW Ms
Uploaded by
Raiyan RahmanCopyright:
Available Formats
ALCOHOLS AND MODERN ANALYTICAL TECHNIQUES HW MS
1.
(a)
(i)
(ii)
(volatile components) can escape/distil out (1)
ethanal is most volatile/bpt less than 60 C/partial oxidation (1)
(volatile components) cannot escape/ refluxed (1)
complete oxidation will be achieved/oxidised to the acid (1)
C2H5OH + 2[O] CH3COOH + H2O
C2H5OH, 2[O] and CH3COOH (1)
rest of equation (1)
(b)
2
[6]
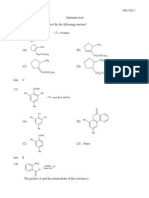
2.
(i)
2-
Cr2O7
(ii)
com pound E
H
H 3C
C H
C H
C 7H
14
O H
H 3C
C H
C H
H 2O
C O O H
C 7H
C
C H
H 3C
2 [O ]
C
H 3C
com pound F
14
(iii)
carboxylic acid would have an absorption between
-1
-1
-1
1680 1750 cm /1700 cm or 2500 3300 cm .
1
[6]
3.
(a)
(b)
(i)
H2SO4 any mention of (aq) loses the mark
(ii)
any correct formula/structure or name for benzoic acid
(i)
dichromate/Cr2O7 /permanganate
(ii)
1
O H
+ [O ]
H
C
H
C
O H
H 2C
C H
H 2C
C H
C
H
H 2C
C H
C 6H
C
H
12
+ [O ]
C 6H
H 2O
H 2O
H 2C
+ [O ]
2
10
H 2O
[4]
Paddington Academy
4.
(a)
Cr2O7
(b)
H+
(i)
2-
(ii)
Orange to green/black/blue
(i)
contains a C=O/aldehyde, ketone, carboxylic acid and ester/
carbonyl/carbonyl in an aldehyde
(ii)
does not contain a OH/ (hydrogen bonded in a) carboxylic acid
(iii)
distillation (no mark) because distillation allows loss of volatile
components /removes butanal from oxidising mixture
prevents formation of RCOOH/ partial oxidation would be achieved
or reverse argument for reflux not being used
in that reflux prevents loss of volatile components
hence complete oxidation would be achieved/RCOOH would be formed
[7]
5.
(a)
(b)
(i)
alkene
alcohol/hydroxy/hydroxyl
(i)
I = alkene & II = alcohol both are needed
(ii)
decolourised / colourless
(iii)
O H
Br
Br
(iv)
X as shown below
T R A N S M IT T A N C E %
100
50
0
4000
3000
2000
1500
1000
500
H AVENU M BER
(c)
(i)
Ni/Pt/Rh/Pd
(ii)
compound B is C10H22O
(iii)
C10H20O H2 C10H22O
1
[9]
Paddington Academy
6.
(a)
(i)
Alkene/C=C
1
-
(ii)
(b)
Alcohol/ROH/hydroxy/hydroxyl/OH (not OH or hydroxide)
One of the C in both C=C is joined to two atoms or groups that
are the same
Observation
decolourisation (of Br2)
Molecular formula
C10H18OBr4
C10H18OBr2 gets 1 mark
(c)
(d)
reagent
CH3COOH
catalyst
H2SO4/H /HCl (aq) or dilute loses the mark
(i)
C10H18O 2[O] C10H16O2 H2O
1
2
1 mark for H2O and 1 mark for 2[O]
(ii)
The infra-red spectrum was of compound Y
-1
because absorption between 1680 1750 cm indicates a C=O
-1
and the absence of a peak between 2500 3300 cm shows the absence
of the OH hydrogen bonded in a carboxylic acid
1
[12]
7.
(i)
(ii)
Any two realistic fragments,
+
+
+
+
+
e.g. CH3 : 15; C2H5 : 29; C3H7 : 43; C4H9 : 57; OH : 17, etc. (1) (1)
Do not penalise missing charge.
breathalysers/monitoring of air pollution, MOT emission testing, etc. (1)
1
[3]
8.
mole ratio = 88.89/12 : 11.1/1 = 7.41 : 11.1 (1)
empirical formula = C2H3 (1)
relative mass of C2H3 = 27.
Mr = 2 29 so molecular formula = C4H6 (1)
X reacts with 2 mol H2 so there are 2 double bonds (1)
Possible structure = 1,3-butadiene /
(1)
[5]
Paddington Academy
You might also like
- Space DynamicsDocument37 pagesSpace Dynamicspurushottam KashyapNo ratings yet
- CARBOXYLIC ACID REACTIONSDocument24 pagesCARBOXYLIC ACID REACTIONSGulshan kumarNo ratings yet
- Saudi Aramco welding inspection planDocument12 pagesSaudi Aramco welding inspection planspravin231No ratings yet
- Geology For Civil EngineersDocument4 pagesGeology For Civil EngineersMr. DummyNo ratings yet
- Produced Water OverviewDocument86 pagesProduced Water Overviewsigit cahyonoNo ratings yet
- Class Test-1-Aldehydes & Ketones - PreparationDocument5 pagesClass Test-1-Aldehydes & Ketones - PreparationSarthak VermaNo ratings yet
- Alcohol, Phenol and Ether Study GuideDocument10 pagesAlcohol, Phenol and Ether Study GuideAbhishek SharmaNo ratings yet
- Section-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XDocument14 pagesSection-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XPriyansh YadavNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Fan Et Al. - Solids Mixing - Ind. and Eng. Chemistry (1970) Vol 62 NR 7Document17 pagesFan Et Al. - Solids Mixing - Ind. and Eng. Chemistry (1970) Vol 62 NR 7BerndUmmeNo ratings yet
- EXP-FR-UT090-EN-R0 - 3 - Fire and FoamDocument143 pagesEXP-FR-UT090-EN-R0 - 3 - Fire and FoamAnonymous aIuHKoKZj100% (1)
- Compact Conductor CalculationDocument3 pagesCompact Conductor CalculationGautama Chandra PradiptaNo ratings yet
- Alcohol AnswersDocument6 pagesAlcohol Answerslucylovesbooks6770No ratings yet
- BT Alcolhols Phenls and Ethers - ExerciseDocument20 pagesBT Alcolhols Phenls and Ethers - ExerciseSanjay KumarNo ratings yet
- Equation Name of Mechanism Mechanism: 4.8, 4.9 Exam Questions Ms 1Document9 pagesEquation Name of Mechanism Mechanism: 4.8, 4.9 Exam Questions Ms 1Adnan ChowdhuryNo ratings yet
- Alcohol MarkschemeDocument23 pagesAlcohol MarkschemeqwedsaNo ratings yet
- 4.10 - 4.11 Organic Synthesis and Structure Determination MSDocument30 pages4.10 - 4.11 Organic Synthesis and Structure Determination MSAdnan ChowdhuryNo ratings yet
- Analytical Chemistry AQA AnswersDocument30 pagesAnalytical Chemistry AQA AnswersAhmad BustamiNo ratings yet
- Aliphatic HydrocarbonsDocument11 pagesAliphatic HydrocarbonsRishabhNo ratings yet
- Chem Unit 5 Organic AnswersDocument47 pagesChem Unit 5 Organic Answersareyouthere92No ratings yet
- EdExcel A Level Chemistry Unit 4 Mark Scheme Results Paper 1 Jun 2005Document10 pagesEdExcel A Level Chemistry Unit 4 Mark Scheme Results Paper 1 Jun 2005MashiatUddinNo ratings yet
- CL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDocument12 pagesCL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDivya KhandelwalNo ratings yet
- Fiitjee: Are) Y (And) X - Y X (Document3 pagesFiitjee: Are) Y (And) X - Y X (Sankar KumarasamyNo ratings yet
- Alcohol Ether and PhenolsDocument43 pagesAlcohol Ether and Phenolswadhwaniakansha9No ratings yet
- 4.06 - 4.07 Aromatic Chemistry and Amines MSDocument20 pages4.06 - 4.07 Aromatic Chemistry and Amines MSAdnan ChowdhuryNo ratings yet
- 4.4, 4.5 Organic Chemistry Reactions and Isomers TestDocument4 pages4.4, 4.5 Organic Chemistry Reactions and Isomers TestKaren JulaoNo ratings yet
- ProjectDocument17 pagesProjectrudrashah4777No ratings yet
- Chemistry Section 3: Atomic Structure and Chemical BondingDocument4 pagesChemistry Section 3: Atomic Structure and Chemical BondingNishant KumarNo ratings yet
- Physics Uploaded NotesDocument4 pagesPhysics Uploaded NotesVuppala Nagasai eshwar santhoshNo ratings yet
- Structure Identification & POCDocument8 pagesStructure Identification & POCHarshil rawal100% (1)
- Sample Questions For Chemistry 2Bh/2Dh Multiple Choice Test THERMODYNAMICS Q1.Document4 pagesSample Questions For Chemistry 2Bh/2Dh Multiple Choice Test THERMODYNAMICS Q1.nihararmyNo ratings yet
- 4.5 4.7 Equilibria and Acids and Bases MSDocument70 pages4.5 4.7 Equilibria and Acids and Bases MSABFauzyNo ratings yet
- UKChO 2005 Round 1 Mark SchemeDocument8 pagesUKChO 2005 Round 1 Mark SchemeLIU YUEYANG RVHS STU 2018No ratings yet
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSDocument7 pagesALDEHYDES, KETONES AND CARBOXYLIC ACIDSkavitha2511977No ratings yet
- 4.4, 4.5 Exam Questions MsDocument9 pages4.4, 4.5 Exam Questions MsswanderfeildNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Check Your Understanding of CarbohydratesDocument21 pagesCheck Your Understanding of CarbohydratesRamNo ratings yet
- Carbonyl Compund Subjective QuestionsDocument11 pagesCarbonyl Compund Subjective QuestionsVinod AgrawalNo ratings yet
- 4.6, 4.7 Exam Questions MsDocument21 pages4.6, 4.7 Exam Questions Msriditha0% (1)
- Alde & Ket-2&3Document14 pagesAlde & Ket-2&3ayesha sheikhNo ratings yet
- Alcohol, Ether, Phenol - PrintDocument19 pagesAlcohol, Ether, Phenol - PrintShubham Raj100% (2)
- Alcohol, Phenol & Ether: A O CH C HDocument6 pagesAlcohol, Phenol & Ether: A O CH C HJitendra KumarNo ratings yet
- 07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1Document15 pages07 Addition and Condensation of Enols and Enolate Ions (1) .PDF - 1JeetNo ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- 9701 Chemistry: MARK SCHEME For The May/June 2010 Question Paper For The Guidance of TeachersDocument6 pages9701 Chemistry: MARK SCHEME For The May/June 2010 Question Paper For The Guidance of TeachersSumaira AliNo ratings yet
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- 9701 s10 Ms 42Document9 pages9701 s10 Ms 42Sheng Qian YewNo ratings yet
- 202 Practice FinalDocument4 pages202 Practice FinalElaineNo ratings yet
- Haloalkanes MarkschemeDocument23 pagesHaloalkanes MarkschemeqwedsaNo ratings yet
- Acid - BasesDocument35 pagesAcid - BasesSachin SinghalNo ratings yet
- Acidicity Basicity & H - Bonding TautomerismDocument10 pagesAcidicity Basicity & H - Bonding TautomerismRaju SinghNo ratings yet
- Aldehydes & KetonesDocument23 pagesAldehydes & KetonesManthan JhaNo ratings yet
- Chemistry Advanced Level Problem Solving (ALPS-1) - PaperDocument15 pagesChemistry Advanced Level Problem Solving (ALPS-1) - PaperAnanmay ChauhanNo ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- Unit-12-Aldehydes, Ketones-MCQDocument5 pagesUnit-12-Aldehydes, Ketones-MCQArsenal Exploiter RepotsNo ratings yet
- Hydrocarbons A 1Document4 pagesHydrocarbons A 1REJA MUKIB KHANNo ratings yet
- Alcohol SheetDocument25 pagesAlcohol SheethtethddthdhtNo ratings yet
- Carboxylic Acid Properties and ReactionsDocument3 pagesCarboxylic Acid Properties and Reactionsimu4u_786No ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Du Entrance Chemistry 2017Document15 pagesDu Entrance Chemistry 2017Arnav ChakrabortyNo ratings yet
- Aldehyde & Ketonesmdtr68a01 QuizDocument5 pagesAldehyde & Ketonesmdtr68a01 QuizSankar KumarasamyNo ratings yet
- 2015 HSC Chemistry PDFDocument42 pages2015 HSC Chemistry PDFlillianaNo ratings yet
- 34 Alcohols & Ethers - Problems For Practice - Level 1Document14 pages34 Alcohols & Ethers - Problems For Practice - Level 1Abuturab MohammadiNo ratings yet
- WORK BOOK - Exercise in ChemistryDocument28 pagesWORK BOOK - Exercise in ChemistryTikeshwar SharmaNo ratings yet
- Phy3 AstroDocument31 pagesPhy3 AstroÑíãzúr Rãhmáñ0% (1)
- Special WaiverDocument1 pageSpecial WaiverRaiyan RahmanNo ratings yet
- Sup 23 MaleDocument4 pagesSup 23 MaleRaiyan RahmanNo ratings yet
- WWW Chemguide Co Uk Analysis Ir Interpret HTML TopDocument11 pagesWWW Chemguide Co Uk Analysis Ir Interpret HTML TopRaiyan RahmanNo ratings yet
- Chemical Equilibrium2Document4 pagesChemical Equilibrium2Raiyan RahmanNo ratings yet
- Examiners' Report/ Principal Examiner Feedback January 2015Document10 pagesExaminers' Report/ Principal Examiner Feedback January 2015Raiyan RahmanNo ratings yet
- 4.3 Rates A Levels ChemistryDocument18 pages4.3 Rates A Levels ChemistrychwalidNo ratings yet
- Mark Scheme (Results) January 2015: Pearson Edexcel International Advanced Level in Physics (WPH05) Paper 01Document18 pagesMark Scheme (Results) January 2015: Pearson Edexcel International Advanced Level in Physics (WPH05) Paper 01annishNo ratings yet
- WPH04 01 MSC 20150305Document14 pagesWPH04 01 MSC 20150305Raiyan RahmanNo ratings yet
- AS Chemistry - Revision Notes Unit 3 - Introduction To Organic ChemistryDocument6 pagesAS Chemistry - Revision Notes Unit 3 - Introduction To Organic ChemistryRaiyan RahmanNo ratings yet
- A2 Chemistry Answer BookDocument85 pagesA2 Chemistry Answer BookHarrys Oustapasidis100% (3)
- Shapes of Simple Molecules and IonsDocument2 pagesShapes of Simple Molecules and IonsMoustafa SohdyNo ratings yet
- Chemistry DefinitionsDocument15 pagesChemistry DefinitionsRaiyan RahmanNo ratings yet
- Chemical EnergeticsDocument7 pagesChemical EnergeticsRaiyan RahmanNo ratings yet
- Energetics SummaryDocument6 pagesEnergetics SummaryJoshua JacobNo ratings yet
- Empirical Formulae & Molar Mass CalculationsDocument1 pageEmpirical Formulae & Molar Mass CalculationsRaiyan RahmanNo ratings yet
- CHEM2 - Chemistry in Action Definitions To Learn: 6. EnergeticsDocument2 pagesCHEM2 - Chemistry in Action Definitions To Learn: 6. EnergeticsRaiyan RahmanNo ratings yet
- Chemical Bonding 2Document1 pageChemical Bonding 2Raiyan RahmanNo ratings yet
- As Topic 5 Notes - AlkanesDocument5 pagesAs Topic 5 Notes - AlkanesRaiyan RahmanNo ratings yet
- 1 Ionic BondingDocument1 page1 Ionic BondingRaiyan RahmanNo ratings yet
- Structural IsomerismDocument1 pageStructural IsomerismRaiyan RahmanNo ratings yet
- 1.4 NotesDocument9 pages1.4 NotesUmer SalmanNo ratings yet
- As Topic 6 Notes - EnergeticsDocument3 pagesAs Topic 6 Notes - Energeticsrabs006100% (1)
- 1.5 NotesDocument14 pages1.5 NotesshunmugathasonNo ratings yet
- Bonding in Organic Compounds - Organic Synthesis Marks SchemeDocument96 pagesBonding in Organic Compounds - Organic Synthesis Marks SchemeRaiyan RahmanNo ratings yet
- 1.7 Intro Organic ChemDocument2 pages1.7 Intro Organic ChemRaiyan RahmanNo ratings yet
- 1.3 Formulae, Equations and Amounts of Substance: Relative Mass Relative ChargeDocument19 pages1.3 Formulae, Equations and Amounts of Substance: Relative Mass Relative ChargeRaiyan RahmanNo ratings yet
- Formulae, Equations and Amounts of SubstanceDocument1 pageFormulae, Equations and Amounts of SubstanceRaiyan RahmanNo ratings yet
- HEAT TRANSFER LAB REPORTDocument45 pagesHEAT TRANSFER LAB REPORTnighatNo ratings yet
- Xampler HFDocument8 pagesXampler HFAnil ReddyNo ratings yet
- 23 - High Temperature Materials - Torralba PDFDocument70 pages23 - High Temperature Materials - Torralba PDFAnish MahajanNo ratings yet
- Determinación de 3 Alkil 2 Metoxipirazinas en Uvas Mostos y VinosDocument9 pagesDeterminación de 3 Alkil 2 Metoxipirazinas en Uvas Mostos y VinosEmmanuel BonninNo ratings yet
- Unit 2 SolutionsDocument5 pagesUnit 2 SolutionsArchana KumariNo ratings yet
- Semiotica - Roma AntiguaDocument25 pagesSemiotica - Roma Antiguarafasolano1610No ratings yet
- VD-SEAL II Final Report2Document4 pagesVD-SEAL II Final Report2Arturo CordovaNo ratings yet
- Bpharmacy 1 Sem Pharmaceutics 1 Set P 2018Document3 pagesBpharmacy 1 Sem Pharmaceutics 1 Set P 2018Mandlik PritiNo ratings yet
- Spin Valve TransistorDocument19 pagesSpin Valve Transistorajayg2020No ratings yet
- The Astronet Infraestructure RoadmapDocument178 pagesThe Astronet Infraestructure RoadmapALNo ratings yet
- Atividade 8º - Inglês - Semana 22Document3 pagesAtividade 8º - Inglês - Semana 22Maria ClaraNo ratings yet
- Opto Semi Koth0001eDocument17 pagesOpto Semi Koth0001eAnurag ChadhaNo ratings yet
- BIOL1177 SM1 2020 Session 2 ProformaDocument7 pagesBIOL1177 SM1 2020 Session 2 ProformaThisarieNo ratings yet
- k30 Euroline BisDocument16 pagesk30 Euroline BiscyberquasitNo ratings yet
- Discover The Difference Between Glycolysis and Krebs CycleDocument5 pagesDiscover The Difference Between Glycolysis and Krebs Cyclepond_1993No ratings yet
- Anios RHW - Sell Sheet PDFDocument2 pagesAnios RHW - Sell Sheet PDFgadhang dewanggaNo ratings yet
- 08ch1013 Anik Roy Iit KGP Mckinsey ResumeDocument2 pages08ch1013 Anik Roy Iit KGP Mckinsey ResumeAnik RoyNo ratings yet
- Week - 2 - Proyect - 2 - Bgu - Activity 5.telescopesDocument8 pagesWeek - 2 - Proyect - 2 - Bgu - Activity 5.telescopesEunisse Macas Palma100% (1)
- Chaper 2 Definition Classification Force SEDocument8 pagesChaper 2 Definition Classification Force SEMaan Valencia - RevillaNo ratings yet
- PH Ysicsguide: Basic Concepts of Statistical MechanicsDocument14 pagesPH Ysicsguide: Basic Concepts of Statistical MechanicsMNo ratings yet
- HTL Ispat PVT LTD: 428 5.100 TotalDocument1 pageHTL Ispat PVT LTD: 428 5.100 Totalmadhav jadhavNo ratings yet
- Bore-log analysis and interpretationDocument6 pagesBore-log analysis and interpretationKaaviyan ThirunyanamNo ratings yet
- Heat Loss PDFDocument2 pagesHeat Loss PDFJessica ZagitaNo ratings yet