Professional Documents
Culture Documents
Enzyme Activity With Temperature
Uploaded by
Guille PazCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Enzyme Activity With Temperature
Uploaded by
Guille PazCopyright:
Available Formats
Guillermo PazEffect of Temperature on the rate of reaction
24/03/15
THE EFFECT OF TEMPERATURE ON THE
RATE OF REACTION OF SALIVARY
AMYLASE
TITLE
Enzymes are found in living cells where they act as catalysts for the thousands of
chemical reactions taking place. Enzymes have numerous applications that affect
our daily lives, for example they enable us to digest food. The investigation was
completed to show us how temperature effects the activity of the enzyme, salivary
amylase. How does temperature affect the rate of reaction of salivary amylase? The
activity should escalate until reaching a certain temperature, in which the enzyme
will denature.
Method:
1. Place 2ml of saliva is test tube, place the test tube in the water bath with ice to equilibrate.
2. Place 2ml of starch solution in a second test tube and place it in ice to equilibrate.
3. After 5 minutes of equilibrating, put the starch solution in to the saliva test tube.
4. A sample of the reaction mixture is removed with a glass rod every 30 seconds and added to a spot
of iodine solution on a white paper.
5. Record the time taken to reach the end-point, this means when no blue-black or reddish brown
coloration occurs.
6. Repeat stages 1-4 at a series of temperatures as follows:
7. In ice (record temperature of reaction mixture)
20C
30C
40C
50C
60C
70C
Apparatus:
Test tubes
Glass rid
250ml flask or beaker
Measuring cylinder
Stopwatch
500ml beaker
Solution of iodine in potassium iodide
Ice
Guillermo PazEffect of Temperature on the rate of reaction
24/03/15
Variables Table:
Variable
Amount of drops of enzyme into
iodine.
Amount of drops for iodine spot
Time for colour to change
Temperature
Type Of Variable
Control
Control
Dependent
Independent
How To control
Each 30 seconds put 2 drops of
enzyme into iodine. No more.
Make the spot 3 drops big.
Temperatures indicated in the
method.
Risk Assessment & Safety:
Iodine is a harmful substance, be careful when manipulating it. Might want to were gloves and
goggles.
Hot water can be very harmful, never produce the experiment sitting down. Be careful when
transporting or manipulating the hot water.
Ethical and environmental considerations:
The experiment requires a lot of water, try and use the same water for the whole experiment. If this
damages your results, use more than one beaker of water but try and keep the water use to the minimum.
Iodine can be harmful for the environment, keep it in its beaker at all times when not using and be careful
when using it.
Results:
Temperatur
Time
e (degrees
(minutes) Celsius)
11
10
6
20
7
30
9
40
0
50
0
60
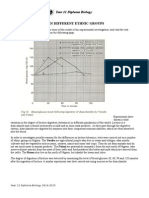
The results in the graph clearly show how at very low temperatures the enzymes are not very effective.
Then as the temperature goes up they start to get larger rate of activity. At very high temperatures (50) the
enzymes denature and do not work anymore.
Conclusions:
Results support the hypothesis stated in the introduction. As the temperature increased the time taken for
the colour of iodine to change colour became lower, this shows enzyme reaction. When the temperature
over past 30/40 degrees Celsius the enzyme reaction was cero, as the colour did not change until the
Guillermo PazEffect of Temperature on the rate of reaction
24/03/15
iodine solution diluted with the high quantities of saliva and starch. This shows that the hypothesis was
correct.
Proposals for extension:
The question might need further research as we used saliva from only two people, meaning we could not
have a wither range of peoples saliva and results. Also there were many errors made during the
experiment.
Modifications and improvements: how the errors and limitations listed previously could be reduced or
eliminated - be scientific and exact in this section.
The experiment explored has a lot of limitations. For example: The type of saliva used for the
experiment. The time of day saliva samples were taken. This was after lunch; food might of
affected the enzyme. The saliva was only picked from two different persons.
Errors: A 30 seconds difference between each sample of saliva and starch taken from the test tube
was a very small window so the amount added to the iodine after minutes was very large, meaning
the solution could easily become dilute. Temperature could not be kept the same, so 5C were
added to the actual temperature experimented. As time passed the temperature decrease to the
temperature needed.
You might also like
- Chem12th Imp Proj PDFDocument8 pagesChem12th Imp Proj PDFKreya ParmarNo ratings yet
- New FileDocument9 pagesNew Filekarthikeyagajula93No ratings yet
- Chemistry Project FinalDocument15 pagesChemistry Project FinalsanjaykumartfguilNo ratings yet
- Blue Simple Illustration Science and Research Stationery A4 Page Border - 20231101 - 192225 - 0000Document16 pagesBlue Simple Illustration Science and Research Stationery A4 Page Border - 20231101 - 192225 - 0000bhabak0024No ratings yet
- Chemistry Project Report OnDocument9 pagesChemistry Project Report OnVarun Jain67% (3)
- Chem Final - PrajithDocument13 pagesChem Final - PrajithA PRAJITH ARWESMENTNo ratings yet
- Temperature and Yeast GrowthDocument4 pagesTemperature and Yeast Growthhunarsandhu33% (6)
- Objectives of The Project Repor1Document5 pagesObjectives of The Project Repor1Sunny SinghNo ratings yet
- To Study The Digestion of Starch by SaliDocument12 pagesTo Study The Digestion of Starch by SaliUthaya SurianNo ratings yet
- To Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On ItDocument4 pagesTo Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On Itethirajmagesh5No ratings yet
- Chemistry Project-1Document11 pagesChemistry Project-1chicku167No ratings yet
- Chemistry Investigatory ProjectDocument18 pagesChemistry Investigatory ProjectWoah NitinNo ratings yet
- Digestion of Salivary GlandDocument12 pagesDigestion of Salivary GlandJatin suthar100% (1)
- Study Digestion of Starch by Salivary AmylaseDocument4 pagesStudy Digestion of Starch by Salivary AmylaseTanishq SainiNo ratings yet
- Harsh ChemistryDocument8 pagesHarsh Chemistryhateu1588No ratings yet
- Chemistry ProjectDocument19 pagesChemistry ProjectKabeer Golechha100% (1)
- Chemistry Investigatory Project - BS12B205Document20 pagesChemistry Investigatory Project - BS12B205JAI PRAKASH M DNo ratings yet
- Effect of pH and Temperature on Starch DigestionDocument18 pagesEffect of pH and Temperature on Starch DigestionUrvit DagarNo ratings yet
- Cool 3D Chemistry Middle School Student Pack by SlidesgoDocument10 pagesCool 3D Chemistry Middle School Student Pack by SlidesgoKreya ParmarNo ratings yet
- Effect of pH and Temperature on Salivary Amylase DigestionDocument27 pagesEffect of pH and Temperature on Salivary Amylase DigestionMahaprasad Manoranjan Sahoo100% (1)
- Chemistry Investigatory ProjectDocument18 pagesChemistry Investigatory ProjectPavan Kumar40% (5)
- Harsh Singh Chemistry3Document10 pagesHarsh Singh Chemistry3hateu1588No ratings yet
- Chemistry Investigatory ProjectDocument16 pagesChemistry Investigatory ProjectSriram .R100% (4)
- Jitendra Sir PGT, Chemistry KV Ghazipur Alok Kumar Bind PGT, Chemistry Class:-Xii Roll No: - Alok Kumar BindDocument12 pagesJitendra Sir PGT, Chemistry KV Ghazipur Alok Kumar Bind PGT, Chemistry Class:-Xii Roll No: - Alok Kumar BindAlex Smith75% (4)
- Digestion Through Saliva AnalysisDocument5 pagesDigestion Through Saliva AnalysisRicky SharmaNo ratings yet
- Chemistry Investigatory File - 063557Document21 pagesChemistry Investigatory File - 063557goelvinayak406No ratings yet
- A ProjectDocument17 pagesA ProjectDivya Yadav100% (1)
- Chemistry Investigatory Project (1) 3Document16 pagesChemistry Investigatory Project (1) 3Manjushree .TNo ratings yet
- Lab #: 4 Date: February 13, 2020 Title: Enzymes Skill: AimDocument3 pagesLab #: 4 Date: February 13, 2020 Title: Enzymes Skill: AimSabrena RowlandNo ratings yet
- Edexcel Biology IGCSE: Enzyme Temperature EffectDocument3 pagesEdexcel Biology IGCSE: Enzyme Temperature EffectahmedNo ratings yet
- Chem Inves Proj FinalDocument13 pagesChem Inves Proj FinalphychemgodNo ratings yet
- Chemistry Project Report OnDocument14 pagesChemistry Project Report OnAditya MaviNo ratings yet
- Chemistry ProjectDocument18 pagesChemistry ProjectmohitNo ratings yet
- Salivary Amylase ProjectDocument14 pagesSalivary Amylase ProjectTanmay SharmaNo ratings yet
- Edexcel IGCSE Biology Practical NotesDocument38 pagesEdexcel IGCSE Biology Practical NotesMinhajul Islam MahinNo ratings yet
- Effect of Temperature and pH on Starch Digestion by Salivary AmylaseDocument19 pagesEffect of Temperature and pH on Starch Digestion by Salivary AmylaseKuldeep GourNo ratings yet
- Phyics ExtraDocument11 pagesPhyics ExtraHimanshu Somkunwar0% (1)
- New Microsoft Word DocumentDocument6 pagesNew Microsoft Word Documentprinsuumrao3625No ratings yet
- 12TH Chem.Document13 pages12TH Chem.Tanu SinghNo ratings yet
- PROJECTDocument14 pagesPROJECTOkay DibzNo ratings yet
- Bio Lab - Enzymes and Temp.Document2 pagesBio Lab - Enzymes and Temp.ZahraNo ratings yet
- Amylase Activity Experiment: Enzymes As Biological CatalystsDocument4 pagesAmylase Activity Experiment: Enzymes As Biological CatalystsUsman AliNo ratings yet
- Chemistry Project Report On To Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On ItDocument5 pagesChemistry Project Report On To Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On ItGSI BHUBANESWARNo ratings yet
- Yeast LabDocument15 pagesYeast LabSteve KimNo ratings yet
- To Study The Digestion of Starch by SalivaDocument15 pagesTo Study The Digestion of Starch by SalivaUrvit Dagar100% (1)
- Chemistryproject - 12Document14 pagesChemistryproject - 12Hitesh MendirattaNo ratings yet
- ChemistryDocument8 pagesChemistryVASUNo ratings yet
- Prakhar Chemistry ProjectDocument12 pagesPrakhar Chemistry ProjectVEDANT ONLYNo ratings yet
- Enzymes LolsDocument38 pagesEnzymes LolsThon JustineNo ratings yet
- Enzymes LolsDocument39 pagesEnzymes LolsA FloraldeNo ratings yet
- Chemistry Investigatory Project Class 12Document6 pagesChemistry Investigatory Project Class 12adhyyansingh458No ratings yet
- ChemistryDocument14 pagesChemistrySHREENo ratings yet
- Bio Lab Final ReportDocument13 pagesBio Lab Final Report햇님No ratings yet
- Bio S5 SBADocument5 pagesBio S5 SBAWONG EVELYNE JADENo ratings yet
- GRADE XII Chemistry ProjectDocument13 pagesGRADE XII Chemistry ProjectroobanNo ratings yet
- Enzyme Pratica 2022Document17 pagesEnzyme Pratica 2022Nguyễn HuyềnNo ratings yet
- Name - Class - 12 Roll No. - Subject - Chemistry Year - 2021-22 SchoolDocument18 pagesName - Class - 12 Roll No. - Subject - Chemistry Year - 2021-22 SchoolDevilNo ratings yet
- Act 5 LabDocument3 pagesAct 5 LabValenzuela Allene GraceNo ratings yet
- Edexcel IGCSE Triple Award Biology Experimental Method NotesDocument17 pagesEdexcel IGCSE Triple Award Biology Experimental Method Notes212852No ratings yet
- Noah's Fascinating World of STEAM Experiments: Chemical Reactions: A Junior Scientist's Lab Notebook for Learning Scientific MethodFrom EverandNoah's Fascinating World of STEAM Experiments: Chemical Reactions: A Junior Scientist's Lab Notebook for Learning Scientific MethodNo ratings yet
- Reativity (The Arts and Other Experiences That Involve Creative Thinking)Document2 pagesReativity (The Arts and Other Experiences That Involve Creative Thinking)Guille PazNo ratings yet
- Group 4 Project 2015 Information BookletDocument16 pagesGroup 4 Project 2015 Information BookletJohn OsborneNo ratings yet
- End of Q2 ReflectionsDocument2 pagesEnd of Q2 ReflectionsGuille PazNo ratings yet
- Hershey and Chase ExperimentDocument1 pageHershey and Chase ExperimentGuille PazNo ratings yet
- Recordin Q2 The RestDocument7 pagesRecordin Q2 The RestGuille PazNo ratings yet
- End of Quimester 2 Summary TablesDocument3 pagesEnd of Quimester 2 Summary TablesGuille PazNo ratings yet
- Matyuschenckos Group 4 Project:: Personal Log Other Group 4 ProjectsDocument8 pagesMatyuschenckos Group 4 Project:: Personal Log Other Group 4 ProjectsGuille PazNo ratings yet
- Bio Lactose TolerantDocument3 pagesBio Lactose TolerantGuille PazNo ratings yet
- March 2015 CalendarDocument2 pagesMarch 2015 CalendarGuille PazNo ratings yet
- Planning Q2: Which Activities Do You Plan To Participate in This Quimester?Document3 pagesPlanning Q2: Which Activities Do You Plan To Participate in This Quimester?Guille PazNo ratings yet
- Summary Enzyme Use To Remove Lactose From MilkDocument1 pageSummary Enzyme Use To Remove Lactose From MilkGuille PazNo ratings yet
- April 2015 CalendarDocument2 pagesApril 2015 CalendarGuille PazNo ratings yet
- July 2015 CalendarDocument1 pageJuly 2015 CalendarGuille PazNo ratings yet
- Recording Q2 PAGE1Document3 pagesRecording Q2 PAGE1Guille PazNo ratings yet
- June 2015 CalendarDocument2 pagesJune 2015 CalendarGuille PazNo ratings yet
- MitosisDocument3 pagesMitosisGuille PazNo ratings yet
- May 2015 CalendarDocument2 pagesMay 2015 CalendarGuille PazNo ratings yet
- End of Q1 ReflectionsDocument2 pagesEnd of Q1 ReflectionsGuille PazNo ratings yet
- Self Reveiw and Personal GoalsDocument4 pagesSelf Reveiw and Personal GoalsGuille PazNo ratings yet
- Planning Q2: Which Activities Do You Plan To Participate in This Quimester?Document4 pagesPlanning Q2: Which Activities Do You Plan To Participate in This Quimester?Guille PazNo ratings yet
- End of Quimester 1 Summary TablesDocument2 pagesEnd of Quimester 1 Summary TablesGuille PazNo ratings yet
- Diagnostic EvaluationDocument14 pagesDiagnostic EvaluationGuille PazNo ratings yet
- Recording Q1Document2 pagesRecording Q1Guille PazNo ratings yet
- Recording Q1Document8 pagesRecording Q1Guille PazNo ratings yet
- Pie Chart For Cancer Frequency in ArgentinaDocument1 pagePie Chart For Cancer Frequency in ArgentinaGuille PazNo ratings yet
- Added Fresh Water %Document1 pageAdded Fresh Water %Guille PazNo ratings yet
- Stem CellsDocument1 pageStem CellsGuille PazNo ratings yet
- Planning Q1Document5 pagesPlanning Q1Guille PazNo ratings yet
- Hoe Does A Tick Get To Its Host?: Guillermo PazDocument5 pagesHoe Does A Tick Get To Its Host?: Guillermo PazGuille PazNo ratings yet
- RJ Bensingh Pub Composites CoatingsDocument5 pagesRJ Bensingh Pub Composites CoatingsFakkir MohamedNo ratings yet
- EPF, EPS, Gratuity, Superannuation & Group Life Insurance Nomination FAQsDocument4 pagesEPF, EPS, Gratuity, Superannuation & Group Life Insurance Nomination FAQsHemanth DhananjayNo ratings yet
- Lesson 8 - Philippine Disaster Risk Reduction and Management SystemDocument11 pagesLesson 8 - Philippine Disaster Risk Reduction and Management SystemMary Joy CuetoNo ratings yet
- Coal Workers' Pneumoconiosis (Black Lung Disease) Treatment & Management - Approach Considerations, Medical Care, Surgical CareDocument2 pagesCoal Workers' Pneumoconiosis (Black Lung Disease) Treatment & Management - Approach Considerations, Medical Care, Surgical CareامينNo ratings yet
- VentilationDocument92 pagesVentilationRobert Nixon100% (1)
- GEH-6680LCI FaultsDocument76 pagesGEH-6680LCI FaultsMuhammad IdreesarainNo ratings yet
- Environmental Science OEdDocument9 pagesEnvironmental Science OEdGenevieve AlcantaraNo ratings yet
- The Turbo Air 6000 Centrifugal Compressor Handbook AAEDR-H-082 Rev 05 TA6000Document137 pagesThe Turbo Air 6000 Centrifugal Compressor Handbook AAEDR-H-082 Rev 05 TA6000Rifki TriAditiya PutraNo ratings yet
- Introduction of MaintenanceDocument35 pagesIntroduction of Maintenanceekhwan82100% (1)
- Capital Cost Mining PDFDocument263 pagesCapital Cost Mining PDFsue1001No ratings yet
- ĐỀ THI THU TNTHPT SỐ 17Document4 pagesĐỀ THI THU TNTHPT SỐ 17Nguyên Hà NguyễnNo ratings yet
- Ionic Equilibrium - DPP 01 (Of Lec 02) - Arjuna JEE 2024Document2 pagesIonic Equilibrium - DPP 01 (Of Lec 02) - Arjuna JEE 2024nrashmi743No ratings yet
- Evolution Chart 3Document1 pageEvolution Chart 3sasupraNo ratings yet
- Batson Et All - 2007 - Anger and Unfairness - Is It Moral Outrage?Document15 pagesBatson Et All - 2007 - Anger and Unfairness - Is It Moral Outrage?Julia GonzalezNo ratings yet
- Hospital & Clinical Pharmacy Q&ADocument22 pagesHospital & Clinical Pharmacy Q&AKrishan KumarNo ratings yet
- Individualized Education PlanDocument7 pagesIndividualized Education PlanElaine Aninang Hupeda100% (2)
- TESC CRC Office & Gym Roof Exterior PaintingDocument6 pagesTESC CRC Office & Gym Roof Exterior PaintinghuasNo ratings yet
- 670W Bifacial Mono PERC ModuleDocument2 pages670W Bifacial Mono PERC Modulemabrouk adouaneNo ratings yet
- Fluvial Erosion Processes ExplainedDocument20 pagesFluvial Erosion Processes ExplainedPARAN, DIOSCURANo ratings yet
- 3-O FaultDocument15 pages3-O FaultJaved Ahmed LaghariNo ratings yet
- Chin Cup Therapy An Effective Tool For The Correction of Class III Malocclusion in Mixed and Late Deciduous DentitionsDocument6 pagesChin Cup Therapy An Effective Tool For The Correction of Class III Malocclusion in Mixed and Late Deciduous Dentitionschic organizerNo ratings yet
- Siemens SIVACON S8, IEC 61439 Switchgear and Control PanelDocument43 pagesSiemens SIVACON S8, IEC 61439 Switchgear and Control PanelGyanesh Bhujade100% (2)
- Baileys in 2009: Case Study Reference No 509-050-1Document17 pagesBaileys in 2009: Case Study Reference No 509-050-1Ernesto KulasinNo ratings yet
- Sugar Reseach in AustraliaDocument16 pagesSugar Reseach in AustraliaJhonattanIsaacNo ratings yet
- Progress of Nanotechnology in Diabetic Retinopathy TreatmentDocument13 pagesProgress of Nanotechnology in Diabetic Retinopathy Treatmentmistic0No ratings yet
- Chapter 2 Electronic StructureDocument62 pagesChapter 2 Electronic StructureLivan TuahNo ratings yet
- Product GuideDocument13 pagesProduct Guidekhalid mostafaNo ratings yet
- 2022 TESAS PublicationDocument103 pages2022 TESAS PublicationNathan LakaNo ratings yet
- Keys To Biblical CounselingDocument7 pagesKeys To Biblical CounselingDavid Salazar100% (6)
- Kloos Community Psychology Book FlyerDocument2 pagesKloos Community Psychology Book FlyerRiska MirantiNo ratings yet