Professional Documents
Culture Documents
GM501 Hiahuronidaza: Mã sản phẩm: 4 HY 0010 Mã sản phẩm: 4 HY 0001-5
Uploaded by
VuPhuongTra0 ratings0% found this document useful (0 votes)
20 views1 pageThis document provides information on GM501 Hyaluronidase, including its intended use, instructions for use, and specifications. GM501 Hyaluronidase is a ready-to-use solution containing 80 IU/ml of bovine-derived hyaluronidase dissolved in HEPES-buffered medium. It is intended to digest the hyaluronic acid in the cumulus oocyte complex and aid in denuding oocytes during IVF procedures. Instructions describe using 100 μl or 400-600 μl of the solution to denude oocytes for 30 seconds before washing. The product is manufactured and tested to specified standards for pH, osmolality, sterility, endotoxins, and ability to support blastocyst development
Original Description:
thụ tinh nhân tạo
Original Title
GM501 Hiahuronidaza
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides information on GM501 Hyaluronidase, including its intended use, instructions for use, and specifications. GM501 Hyaluronidase is a ready-to-use solution containing 80 IU/ml of bovine-derived hyaluronidase dissolved in HEPES-buffered medium. It is intended to digest the hyaluronic acid in the cumulus oocyte complex and aid in denuding oocytes during IVF procedures. Instructions describe using 100 μl or 400-600 μl of the solution to denude oocytes for 30 seconds before washing. The product is manufactured and tested to specified standards for pH, osmolality, sterility, endotoxins, and ability to support blastocyst development
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
20 views1 pageGM501 Hiahuronidaza: Mã sản phẩm: 4 HY 0010 Mã sản phẩm: 4 HY 0001-5
Uploaded by
VuPhuongTraThis document provides information on GM501 Hyaluronidase, including its intended use, instructions for use, and specifications. GM501 Hyaluronidase is a ready-to-use solution containing 80 IU/ml of bovine-derived hyaluronidase dissolved in HEPES-buffered medium. It is intended to digest the hyaluronic acid in the cumulus oocyte complex and aid in denuding oocytes during IVF procedures. Instructions describe using 100 μl or 400-600 μl of the solution to denude oocytes for 30 seconds before washing. The product is manufactured and tested to specified standards for pH, osmolality, sterility, endotoxins, and ability to support blastocyst development
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
GM501 Hiahuronidaza
Nguyn liu trong gi sn phm:
M sn phm:
4 HY 0010
1 x 10 ml GM501 Hiahuronidaza
M sn phm:
4 HY 0001-5
5 x 1 ml GM501 Hiahuronidaza
Nguyn liu khng c trong gi sn
phm:
Lng p ( nhit 37C)
a Petri
ng pipet
LAF-bench (ISO5 environment)
Mineral Oil
(e.g. GM501 Mineral Oil)
Washing medium
(e.g. GM501 Wash)
Composition:
80 IU/ml pharmaceutical grade
Hiahuronidaza from bovine origin
solved in HEPES-buffered medium.
Intended use/Intended users:
GM501 Hiahuronidaza is a readytouse
solution designed to digest the
extra cellular matrix of hyaluronic
acid in the cumulus oocyte complex.
The intended users are IVF
professionals
(lab technicians, embryologists
or medical doctors).
Instructions for use:
GM501 Hiahuronidaza contains
HEPES;
no CO2 incubation is required,
just warm it up to 37C.
Depending on the dishes, 100 l
(micodrops) or 400-600 l (cen
ter-/4-well) of GM501 Hiahuronidaza
should be used for
denudation.
The dishes should be covered with
Mineral Oil (e.g.
GM501 Mineral Oil).
Prepare 3-5 droplets (100 l) of
washing medium (e.g.
GM501 Wash) for oocyte washing (all
under mineral oil,
e.g. GM501 Mineral Oil).
Place the oocyte in the Hiahuronidaza
(up to max. 5 oocytes)
for about 30 seconds.
Using a fine pipette, transfer the
partially denuded oocytes
to the first washing droplet.
Remove the corona by pipetting the
oocytes.
Use the other droplets to further
wash the denuded oocyte.
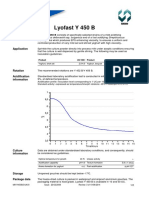
Product specifications and quality
control:
All raw materials are of highest
available purity (European
Pharmacopoeia and/or USP standard), if
applicable.
A certificate of analysis is available
for each batch upon
request from our website with respective
lot number.
The MSDS for GM501 Hiahuronidaza
is available upon
request and can also be downloaded from
our website.
GM501 Hiahuronidaza is
manufactured and tested according
to the following specifications
cenpH
(at 37C) 7.20-7.60
Osmolality (mOsm/kg) 270-290
Sterility sterile - SAL 10-3 (Sterility
Assurance Level)
Endotoxins (EU/ml) < 1.00
MEA (Blastocysts after 96h in %)
Precautions and warnings:
Standard measures to prevent
infections resulting from the
use of medicinal products prepared from
human blood or
plasma include selection of donors,
screening of individual
donations and plasma pools for specific
markers of infection
and the inclusion of effective
manufacturing steps for
the inactivation/removal of viruses.
Despite this, when medicinal
products prepared from human blood or
plasma are
administered, the possibility of
transmitting infective agents
cannot totally be excluded. This also
applies to unknown or
emerging viruses and other pathogens.
There are no reports
of proven virus transmissions with
albumin, manufactured
to European Pharmacopoeia
specifications by established
processes.
Therefore, handle all specimens as if
capable of transmitting
HIV or hepatitis. Always wear protective
clothing when
handling specimens.
Always work under strict hygienic
conditions (LAF-bench
ISO Class 5) to avoid possible
contamination.
Only for the intended use.
Pre-use checks:
Do not use the product if seal of the
container is opened or
defect when the product is delivered.
Do not use the product if it becomes
discoloured, cloudy or
shows any evidence of microbial
contamination.
You might also like
- K. Cameron Campbell MD: Lab DirectorDocument1 pageK. Cameron Campbell MD: Lab Directorjimena ramirezNo ratings yet
- Dr. Ali's Uworld Notes For Step 2 CKDocument40 pagesDr. Ali's Uworld Notes For Step 2 CKmarina shawkyNo ratings yet
- PreciControl Multimarker - Ms - 05341787190.v4.en PDFDocument2 pagesPreciControl Multimarker - Ms - 05341787190.v4.en PDFARIF AHAMMED P100% (1)
- Science of Vaccine DamageDocument35 pagesScience of Vaccine DamageLeonard MichlinNo ratings yet
- Sexually Transmitted DiseasesDocument3 pagesSexually Transmitted Diseasesapi-26570979100% (2)
- Loratadine 5Mg/5Ml Syrup PL 04917/0067Document20 pagesLoratadine 5Mg/5Ml Syrup PL 04917/0067Hanung Puspita Aditya SNo ratings yet
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3Document26 pagesLyphochek Immunoassay Plus Control Levels 1, 2 and 3Aniket dubeyNo ratings yet
- Infection Control and Safety MeasuresDocument29 pagesInfection Control and Safety MeasuresAlma Susan100% (1)
- Nursing Care PlanDocument4 pagesNursing Care PlanJoy Callo100% (2)
- Medical Waste ManagementDocument35 pagesMedical Waste ManagementSloy Semo100% (3)
- Growth Hormone HGH - IMMULITE 2000 Systems - Rev 11 2018Document33 pagesGrowth Hormone HGH - IMMULITE 2000 Systems - Rev 11 2018Mario Echeverria GonzalezNo ratings yet
- Manual G5 INTL v5-0-5Document68 pagesManual G5 INTL v5-0-5ranachamanNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2Document16 pagesLyphochek Assayed Chemistry Control Levels 1 and 2PATH LABNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2Document4 pagesLyphochek Assayed Chemistry Control Levels 1 and 2Gaurav MauryaNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2Document19 pagesLyphochek Assayed Chemistry Control Levels 1 and 2minhasdanial31No ratings yet
- Pglu-Pap GB-D 21 001Document4 pagesPglu-Pap GB-D 21 001akaweadeNo ratings yet
- Cetrotide Epar Scientific Discussion enDocument12 pagesCetrotide Epar Scientific Discussion enUmair MazharNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2Document4 pagesLyphochek Assayed Chemistry Control Levels 1 and 2Gaurav MauryaNo ratings yet
- #C-310-5 Lot.89740 EinsertDocument3 pages#C-310-5 Lot.89740 Einsertjnyng222100% (2)
- Kit InsertDocument2 pagesKit InsertfaujraneNo ratings yet
- Inserto ThermofisherDocument40 pagesInserto ThermofisherConstanza Salinas NuñezNo ratings yet
- Haptoglobin ARC CHEMDocument8 pagesHaptoglobin ARC CHEMbassam alharaziNo ratings yet
- Haemoglobin: Colorimetric Method (Cyanmethemoglobin)Document2 pagesHaemoglobin: Colorimetric Method (Cyanmethemoglobin)Rury Darwa NingrumNo ratings yet
- at Gamma GTDocument2 pagesat Gamma GTNia MarianiNo ratings yet
- Sacco 450b Grafico PH Vs TDocument2 pagesSacco 450b Grafico PH Vs TLaura Vanessa AriasNo ratings yet
- Biorad Lyphocheck Package Leaflet 26460Document2 pagesBiorad Lyphocheck Package Leaflet 26460Retno MonikaNo ratings yet
- Dade Thrombin ReagentDocument8 pagesDade Thrombin ReagentchaiNo ratings yet
- Package Insert - 06038 - HCG - en - 30405 Europa PDFDocument7 pagesPackage Insert - 06038 - HCG - en - 30405 Europa PDFadybaila4680No ratings yet
- 30 405-01 Vidas HCG: Principle of The ProcedureDocument12 pages30 405-01 Vidas HCG: Principle of The ProcedureCitra LatisiaNo ratings yet
- Laboratory Procedure Manual: Triglycerides SerumDocument22 pagesLaboratory Procedure Manual: Triglycerides SerumTarunNo ratings yet
- Trigly F MetDocument22 pagesTrigly F MetdamirNo ratings yet
- Precinorm - Precipath Fructosamine.11934589001.V9.en PDFDocument2 pagesPrecinorm - Precipath Fructosamine.11934589001.V9.en PDFARIF AHAMMED PNo ratings yet
- GMP Haccp enDocument8 pagesGMP Haccp enPedro torres touchNo ratings yet
- Lab Policies Beta HCG - Cobas E601 Lab 4005Document5 pagesLab Policies Beta HCG - Cobas E601 Lab 4005Marj MendezNo ratings yet
- IfU ATG1010engl, DT, Es-18062014 Ab Lot 016Document23 pagesIfU ATG1010engl, DT, Es-18062014 Ab Lot 016f.baxyNo ratings yet
- MP HT 19343yuDocument2 pagesMP HT 19343yuVanessa Rojas LoyolaNo ratings yet
- Advate 2005 EPAR Scientific DiscussionDocument44 pagesAdvate 2005 EPAR Scientific DiscussionbioNo ratings yet
- Igf ElisaDocument10 pagesIgf Elisadidit satmokoNo ratings yet
- Small Volume Parentrals: Dr.Y.Anand KumarDocument25 pagesSmall Volume Parentrals: Dr.Y.Anand Kumarsaloni patelNo ratings yet
- Resources-Hematology-Para 12 Plus-04 Instructions (IFU) - 01 IFU para 12 Plus IFUDocument9 pagesResources-Hematology-Para 12 Plus-04 Instructions (IFU) - 01 IFU para 12 Plus IFUpepecalienes2052No ratings yet
- Myoglobin ELISA: For The Quantitative Determination of Myoglobin in Human SerumDocument8 pagesMyoglobin ELISA: For The Quantitative Determination of Myoglobin in Human SerumTanveerNo ratings yet
- CHR Hansen Dry Rennet SachetsDocument4 pagesCHR Hansen Dry Rennet SachetsRegilio MartoNo ratings yet
- PDFDocument2 pagesPDFAde FeriyatnaNo ratings yet
- MH144 SJSMSNDocument3 pagesMH144 SJSMSNsulistyani sapardiNo ratings yet
- Microbial Contamination and GMP-Second LectureDocument22 pagesMicrobial Contamination and GMP-Second Lecturenadesansudhakar100% (1)
- Delfia Hafp A004-201 enDocument21 pagesDelfia Hafp A004-201 enJonny MossNo ratings yet
- Biuret-Gornall Protein Assay: Product DescriptionDocument3 pagesBiuret-Gornall Protein Assay: Product Descriptioninsiya insiyaNo ratings yet
- Public Assessment Report Scientific Discussion Ciclosporin Pharmachemie Capsules 25/50/100mg (Ciclosporin)Document6 pagesPublic Assessment Report Scientific Discussion Ciclosporin Pharmachemie Capsules 25/50/100mg (Ciclosporin)rose goldNo ratings yet
- GBDocument14 pagesGBQuoctytranNo ratings yet
- #694 Lot.45980 EinsertDocument2 pages#694 Lot.45980 Einsertjnyng222No ratings yet
- For Aflatoxin Quantitative Test: Product Number: 8030Document12 pagesFor Aflatoxin Quantitative Test: Product Number: 8030Ceci PelucheNo ratings yet
- Jurnal Stabilitas Suhu PCTDocument7 pagesJurnal Stabilitas Suhu PCTAdnanNo ratings yet
- Insulin ELISA: Instructions For UseDocument9 pagesInsulin ELISA: Instructions For UseJosue Rojas ArayaNo ratings yet
- Normetanephrine Plasma ELISA: Instructions For UseDocument17 pagesNormetanephrine Plasma ELISA: Instructions For UseYousra ZeidanNo ratings yet
- Pectinase 831LDocument1 pagePectinase 831LVeronica V. PortillaNo ratings yet
- MP HT 19344yuDocument2 pagesMP HT 19344yuVanessa Rojas LoyolaNo ratings yet
- R1603 QUICK Histamin 08-02.20Document14 pagesR1603 QUICK Histamin 08-02.20Geraldine Pardo MariluzNo ratings yet
- Rshakmgp-011r Glp-1 (Active) Elisa Kit Eng.001Document18 pagesRshakmgp-011r Glp-1 (Active) Elisa Kit Eng.001scribdNo ratings yet
- Vidas Anti-Hav TotalDocument9 pagesVidas Anti-Hav TotalcassNo ratings yet
- 1551f Veratox For Fumonisin 8830 8831 KitinsertDocument12 pages1551f Veratox For Fumonisin 8830 8831 KitinsertMartin BoninfanteNo ratings yet
- B29061KDocument13 pagesB29061KMeethuanNo ratings yet
- Growth Hormone (HGH)Document33 pagesGrowth Hormone (HGH)Juan Solis Quiroz100% (1)
- Dynabeads Protein G: Product Contents Required Materials ProtocolDocument2 pagesDynabeads Protein G: Product Contents Required Materials ProtocolHakkı SaraylıkNo ratings yet
- Streptavidin HP Spintrap: Ge HealthcareDocument20 pagesStreptavidin HP Spintrap: Ge HealthcareMike BudimanNo ratings yet
- WC 500029819Document23 pagesWC 500029819nsk79in@gmail.comNo ratings yet
- AlphaganDocument9 pagesAlphaganbangunngawiNo ratings yet
- (Pha6114 Lec) 1ST Shifting ReviewerDocument88 pages(Pha6114 Lec) 1ST Shifting ReviewerAndrei RoqueNo ratings yet
- Enfermedades de Tranms. AlimentariaDocument8 pagesEnfermedades de Tranms. AlimentariaAlejandra Dominguez RazoNo ratings yet
- Factsheet Covid 19 Environmental CleaningDocument2 pagesFactsheet Covid 19 Environmental CleaningsNo ratings yet
- Quarter 1 Perform Hand SpaDocument10 pagesQuarter 1 Perform Hand SpaDrexler LabagalaNo ratings yet
- Microbiology (Chapter - Medical Microbiology) Solved MCQs (Set-2)Document5 pagesMicrobiology (Chapter - Medical Microbiology) Solved MCQs (Set-2)Sufiyan AbduramanNo ratings yet
- Ebola Virus Disease: Key FactsDocument2 pagesEbola Virus Disease: Key FactsNi-el 'ndra' SantosoNo ratings yet
- Andrian Hartanto Wijaya - 009201900053 - FinalTestPublicRelationDocument2 pagesAndrian Hartanto Wijaya - 009201900053 - FinalTestPublicRelationirvan suwandiNo ratings yet
- Journal Pre-ProofDocument58 pagesJournal Pre-Proof묭No ratings yet
- URIT 5500 Opeation Manual (V2 0 2012 12 20Document158 pagesURIT 5500 Opeation Manual (V2 0 2012 12 20Paulo sousaNo ratings yet
- ماتريال المراكز والوحدات كاملة-3Document33 pagesماتريال المراكز والوحدات كاملة-3Sanaa SbdelghanyNo ratings yet
- Product Preview 2Document20 pagesProduct Preview 2Dong Zhang100% (1)
- Humss ResearchDocument45 pagesHumss ResearchFRANCHESKA IMIE R. CORILLANo ratings yet
- 1.1.1 How To Stop COVID-19 From Coming On BoardDocument4 pages1.1.1 How To Stop COVID-19 From Coming On BoardMikee MoganNo ratings yet
- Worksheet 1.1 Department of Health Area Program-Based Health PlanningDocument7 pagesWorksheet 1.1 Department of Health Area Program-Based Health PlanningNik Rose ElNo ratings yet
- East & North Herts - Drug Calculation Test-1.Document5 pagesEast & North Herts - Drug Calculation Test-1.nasimhs0% (1)
- Predicting and Controlling Influenza Outbreaks - Published Article - IJERSTE - Vol.12 Issue 2, Feb 2023Document4 pagesPredicting and Controlling Influenza Outbreaks - Published Article - IJERSTE - Vol.12 Issue 2, Feb 2023dimple kharwarNo ratings yet
- Fundamentals in NursingDocument9 pagesFundamentals in NursingWendy EscalanteNo ratings yet
- ANA CDC Whitepaper AntibiotikDocument14 pagesANA CDC Whitepaper AntibiotikKomite PPI RSUDPCNo ratings yet
- CDC & Covid-19 TimelineDocument35 pagesCDC & Covid-19 TimelineKamran ÇələbiyevNo ratings yet
- 413 Health Care SQPDocument7 pages413 Health Care SQPAnil SarohaNo ratings yet
- Sts File To PrintDocument59 pagesSts File To Printshiella mae baltazarNo ratings yet
- Lesson 1 - Microbial ControlDocument3 pagesLesson 1 - Microbial ControlA CNo ratings yet
- Caseous Lymphadenitis (CL) in Goats and SheepDocument4 pagesCaseous Lymphadenitis (CL) in Goats and SheepMorad ImadNo ratings yet