Professional Documents
Culture Documents
Atkin 12001 Molekular Spectroscopy 004
Uploaded by
Nikke ArdilahCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atkin 12001 Molekular Spectroscopy 004
Uploaded by
Nikke ArdilahCopyright:
Available Formats
456
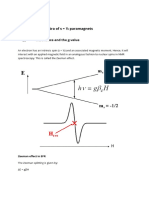
12 MOLECULAR SPECTROSCOPY 1: ROTATIONAL AND VIBRATIONAL SPECTRA
Field
on
MJ
0
1
2
3
Field
off
4
5
7
Fig. 12.12 The effect of an electric eld on
the energy levels of a polar linear rotor.
All levels are doubly degenerate except that
with MJ = 0.
change the moments of inertia (Fig. 12.13). The effect of centrifugal distortion on a
diatomic molecule is to stretch the bond and hence to increase the moment of inertia.
As a result, centrifugal distortion reduces the rotational constant and consequently
the energy levels are slightly closer than the rigid-rotor expressions predict. The effect
is usually taken into account largely empirically by subtracting a term from the energy
and writing
( J) = J(J + 1) J J 2(J + 1)2
Rotational terms affected
by centrifugal distortion
(12.16)
The parameter J is the centrifugal distortion constant. It is large when the bond is
easily stretched. The centrifugal distortion constant of a diatomic molecule is related
to the vibrational wavenumber of the bond, # (which, as we shall see later, is a measure of its stiffness), through the approximate relation (see Problem 12.21)
J =
43
#2
Centrifugal distortion
constant
(12.17)
Hence the observation of the convergence of the rotational levels as J increases can be
interpreted in terms of the rigidity of the bond.
12.5 Rotational transitions
Centrifugal
force
Fig. 12.13 The effect of rotation on a
molecule. The centrifugal force arising
from rotation distorts the molecule,
opening out bond angles and stretching
bonds slightly. The effect is to increase the
moment of inertia of the molecule and
hence to decrease its rotational constant.
Key points (a) For a molecule to give a pure rotational spectrum, it must be polar. The specic
rotational selection rules are J = 1, MJ = 0, 1, K = 0. (b) Bond lengths may be obtained from
analysis of microwave spectra.
Typical values of for small molecules are in the region of 0.110 cm1 (for example,
0.356 cm1 for NF3 and 10.59 cm1 for HCl), so rotational transitions lie in the microwave region of the spectrum. The transitions are detected by monitoring the net absorption of microwave radiation. Modulation of the transmitted intensity, which is used
to facilitate detection and amplication of the absorption, can be achieved by varying
the energy levels with an oscillating electric eld. In this Stark modulation, an electric
eld of about 105 V m1 and a frequency of 10100 kHz is applied to the sample.
(a) Rotational selection rules
We have already remarked (Section 12.2) that the gross selection rule for the observation of a pure rotational spectrum is that a molecule must have a permanent electric
dipole moment. That is, for a molecule to give a pure rotational spectrum, it must be
polar. The classical basis of this rule is that a polar molecule appears to possess a
uctuating dipole when rotating but a nonpolar molecule does not (Fig. 12.14). The
permanent dipole can be regarded as a handle with which the molecule stirs the
electromagnetic eld into oscillation (and vice versa for absorption). Homonuclear
diatomic molecules and symmetrical linear molecules such as CO2 are rotationally
inactive. Spherical rotors cannot have electric dipole moments unless they become
distorted by rotation, so they are also inactive except in special cases. An example of
a spherical rotor that does become sufciently distorted for it to acquire a dipole
moment is SiH4, which has a dipole moment of about 8.3 D by virtue of its rotation
when J 10 (for comparison, HCl has a permanent dipole moment of 1.1 D; molecular dipole moments and their units are discussed in Section 17.1). The pure rotational
spectrum of SiH4 has been detected by using long path lengths (10 m) through highpressure (4 atm) samples.
12.5 ROTATIONAL TRANSITIONS
457
A brief illustration
Of the molecules N2, CO2, OCS, H2O, CH2=CH2, and C6H6, only OCS and H2O are
polar, so only these two molecules have microwave spectra.
Self-test 12.3 Which of the molecules H2, NO, N2O, and CH4 can have a pure
[NO, N2O]
rotational spectrum?
The specic rotational selection rules are found by evaluating the transition dipole
moment between rotational states. We show in Further information 12.2 that, for a
linear molecule, the transition moment vanishes unless the following conditions are
fullled:
J = 1
Rotational selection
rules for linear rotors
MJ = 0, 1
(12.18)
The transition J = +1 corresponds to absorption and the transition J = 1 corresponds to emission. The allowed change in J in each case arises from the conservation
of angular momentum when a photon, a spin-1 particle, is emitted or absorbed
(Fig. 12.15).
When the transition moment is evaluated for all possible relative orientations of
the molecule to the line of ight of the photon, it is found that the total J + 1 J
transition intensity is proportional to
A J+1 D 2
E 0
| J+1,J |2 = B
C 2J + 1 F
(12.19)
where 0 is the permanent electric dipole moment of the molecule. The intensity is
proportional to the square of the permanent electric dipole moment, so strongly polar
molecules give rise to much more intense rotational lines than less polar molecules.
For symmetric rotors, an additional selection rule states that K = 0. To understand
this rule, consider the symmetric rotor NH3, where the electric dipole moment lies
parallel to the gure axis. Such a molecule cannot be accelerated into different states
of rotation around the gure axis by the absorption of radiation, so K = 0. Therefore,
for symmetric rotors the selection rules are:
J = 1
MJ = 0, 1
Fig. 12.14 To a stationary observer,
a rotating polar molecule looks like
an oscillating dipole that can stir the
electromagnetic eld into oscillation
(and vice versa for absorption). This
picture is the classical origin of the gross
selection rule for rotational transitions.
K = 0
Rotational selection rules
for symmetric rotors
(12.20)
(b) The appearance of rotational spectra
When these selection rules are applied to the expressions for the energy levels of a rigid
spherical or linear rotor, it follows that the wavenumbers of the allowed J + 1 J
absorptions are
#(J + 1 J) = (J + 1) ( J) = 2( J + 1)
J = 0, 1, 2, . . .
(12.21a)
When centrifugal distortion is taken into account, the corresponding expression
obtained from eqn 12.16 is
#(J + 1 J) = 2(J + 1) 4J(J + 1)3
(12.21b)
However, because the second term is typically very small compared with the rst, the
appearance of the spectrum closely resembles that predicted from eqn 12.21a.
Photon
Fig. 12.15 When a photon is absorbed by
a molecule, the angular momentum of
the combined system is conserved. If the
molecule is rotating in the same sense as
the spin of the incoming photon, then J
increases by 1.
458
12 MOLECULAR SPECTROSCOPY 1: ROTATIONAL AND VIBRATIONAL SPECTRA
Example 12.3 Predicting the appearance of a rotational spectrum
Predict the form of the rotational spectrum of 14NH3.
Energy
Method We calculated the energy levels in Example 12.2. The 14NH3 molecule is
a polar symmetric rotor, so the selection rules J = 1 and K = 0 apply. For
absorption, J = +1 and we can use eqn 12.21a.
Answer Because = 9.977 cm1, we can draw up the following table for the J + 1
J transitions.
Transmittance
Frequency
Fig. 12.16 The rotational energy levels of a
linear rotor, the transitions allowed by the
selection rule J = 1, and a typical pure
rotational absorption spectrum (displayed
here in terms of the radiation transmitted
through the sample). The intensities reect
the populations of the initial level in each
case and the strengths of the transition
dipole moments.
J
#/cm1
/GHz
0
19.95
598.1
1
39.91
1197
2
59.86
1795
3
79.82
2393
...
...
...
The line spacing is 19.95 cm1 (598.1 GHz).
Self-test 12.4 Repeat the problem for C35ClH3 (see Self-test 12.2 for details).
[Lines of separation 0.944 cm1 (28.3 GHz)]
The form of the spectrum predicted by eqn 12.21 is shown in Fig. 12.16. The most
signicant feature is that it consists of a series of lines with wavenumbers 2, 4, 6,
. . . and of separation 2. The measurement of the line spacing gives , and hence
the moment of inertia perpendicular to the principal axis of the molecule. Because
the masses of the atoms are known, it is a simple matter to deduce the bond length of
a diatomic molecule. However, in the case of a polyatomic molecule such as OCS or
NH3, the analysis gives only a single quantity, I, and it is not possible to infer both
bond lengths (in OCS) or the bond length and bond angle (in NH3). This difculty
can be overcome by using isotopically substituted molecules, such as ABC and ABC;
then, by assuming that R(AB) = R(AB), both AB and BC bond lengths can be
extracted from the two moments of inertia. A famous example of this procedure is the
study of OCS; the actual calculation is worked through in Problem 12.7. The assumption that bond lengths are unchanged by isotopic substitution is only an approximation, but it is a good approximation in most cases. Nuclear spin, which differs from
one isotope to another, also affects the appearance of high-resolution rotational spectra because spin is a source of angular momentum and can couple with the rotation of
the molecule itself and hence affect the rotational energy levels.
The intensities of spectral lines increase with increasing J and pass through a maximum before tailing off as J becomes large. The most important reason for the maximum in intensity is the existence of a maximum in the population of rotational levels.
The Boltzmann distribution (Fundamentals F.5) implies that the population of each
state decays exponentially with increasing J, but the degeneracy of the levels increases,
and these two opposite trends result in the population of the energy levels (as distinct
from the individual states) passing through a maximum. Specically, the population
of a rotational energy level J is given by the Boltzmann expression
NJ NgJ eEJ /kT
where N is the total number of molecules and gJ is the degeneracy of the level J. The
value of J corresponding to a maximum of this expression is found by treating J as
a continuous variable, differentiating with respect to J, and then setting the result
equal to zero. The result is (see Problem 12.26)
A kT D
E
Jmax B
C 2hc F
1/2
1
2
(12.22)
12.6 ROTATIONAL RAMAN SPECTRA
459
For a typical molecule (for example, OCS, with = 0.2 cm1) at room temperature,
kT 1000hc, so Jmax 30. However, it must be recalled that the intensity of each
transition also depends on the value of J (eqn 12.19) and on the population difference
between the two states involved in the transition. Hence the value of J corresponding
to the most intense line is not quite the same as the value of J for the most highly
populated level.
12.6 Rotational Raman spectra
Key points A molecule must be anisotropically polarizable for it to be rotationally Raman active.
The specic selection rules are: (i) linear rotors, J = 0, 2; (ii) symmetric rotors, J = 0, 1, 2;
K = 0.
The gross selection rule for rotational Raman transitions is that the molecule must be
anisotropically polarizable. We begin by explaining what this means. A formal derivation of this rule is given in Further information 12.2.
The distortion of a molecule in an electric eld is determined by its polarizability,
(Section 17.2). More precisely, if the strength of the eld is E, then the molecule
acquires an induced dipole moment of magnitude
= E
(12.23)
in addition to any permanent dipole moment it may have. An atom is isotropically
polarizable. That is, the same distortion is induced whatever the direction of the
applied eld. The polarizability of a spherical rotor is also isotropic. However, nonspherical rotors have polarizabilities that do depend on the direction of the eld
relative to the molecule, so these molecules are anisotropically polarizable (Fig. 12.17).
The electron distribution in H2, for example, is more distorted when the eld is
applied parallel to the bond than when it is applied perpendicular to it, and we write
|| > .
All linear molecules and diatomics (whether homonuclear or heteronuclear) have
anisotropic polarizabilities, and so are rotationally Raman active. This activity is one
reason for the importance of rotational Raman spectroscopy, for the technique can be
used to study many of the molecules that are inaccessible to microwave spectroscopy.
Spherical rotors such as CH4 and SF6, however, are rotationally Raman inactive as well
as microwave inactive. This inactivity does not mean that such molecules are never
found in rotationally excited states. Molecular collisions do not have to obey such
restrictive selection rules, and hence collisions between molecules can result in the
population of any rotational state.
We show in Further information 12.2 that the specic rotational Raman selection
rules are
Linear rotors:
J = 0, 2
Symmetric rotors:
J = 0, 1, 2;
K = 0
Rotational Raman
selection rules
(12.24)
The J = 0 transitions do not lead to a shift in frequency of the scattered photon in pure
rotational Raman spectroscopy, and contribute to the unshifted Rayleigh radiation.
We can predict the form of the Raman spectrum of a linear rotor by applying the
selection rule J = 2 to the rotational energy levels (Fig. 12.18). When the molecule
makes a transition with J = +2, the scattered radiation leaves the molecule in a higher
rotational state, so the wavenumber of the incident radiation, initially #i, is decreased.
These transitions account for the Stokes lines in the spectrum:
E
(a)
E
Distortion
(b)
Fig. 12.17 An electric eld applied to a
molecule results in its distortion, and the
distorted molecule acquires a contribution
to its dipole moment (even if it is nonpolar
initially). The polarizability may be different
when the eld is applied (a) parallel or
(b) perpendicular to the molecular axis (or,
in general, in different directions relative
to the molecule); if that is so, then the
molecule has an anisotropic polarizability.
460
12 MOLECULAR SPECTROSCOPY 1: ROTATIONAL AND VIBRATIONAL SPECTRA
#(J + 2 J) = #i {(J + 2) (J)} = #i 2(2J + 3)
Energy
The Stokes lines appear to low frequency of the incident radiation and at displacements 6, 10, 14, . . . from #i for J = 0, 1, 2, . . . . When the molecule makes a
transition with J = 2, the scattered photon emerges with increased energy. These
transitions account for the anti-Stokes lines of the spectrum:
Signal
Rayleigh line
#(J 2 J) = #i {(J) (J 2)} = #i + 2(2J 1)
Stokes
lines
Anti-Stokes
lines
(12.25b)
The anti-Stokes lines occur at displacements of 6, 10, 14, . . . (for J = 2, 3, 4, . . . ;
J = 2 is the lowest state that can contribute under the selection rule J = 2) to high frequency of the incident radiation. The separation of adjacent lines in both the Stokes
and the anti-Stokes regions is 4, so from its measurement I can be determined and
then used to nd the bond lengths exactly as in the case of microwave spectroscopy.
Example 12.4 Predicting the form of a Raman spectrum
Predict the form of the rotational Raman spectrum of 14N2, for which = 1.99 cm1,
when it is exposed to 336.732 nm laser radiation.
Frequency
The rotational energy levels of
a linear rotor and the transitions allowed
by the J = 2 Raman selection rules.
The form of a typical rotational Raman
spectrum is also shown. The Rayleigh
line is much stronger than depicted in
the gure; it is shown as a weaker line to
improve visualization of the Raman lines.
Fig. 12.18
(12.25a)
Method The molecule is rotationally Raman active because end-over-end rotation
modulates its polarizability as viewed by a stationary observer. The Stokes and
anti-Stokes lines are given by eqn 12.25.
Answer Because i = 336.732 nm corresponds to #i = 29 697.2 cm1, eqns 12.25a
and 12.25b give the following line positions:
J
Stokes lines
#/cm1
/nm
Anti-Stokes lines
#/cm1
/nm
29 685.3
336.868
29 677.3
336.958
29 669.3
337.048
29 661.4
337.139
29 709.1
336.597
29 717.1
336.507
There will be a strong central line at 336.732 nm accompanied on either side by
lines of increasing and then decreasing intensity (as a result of transition moment
and population effects). The spread of the entire spectrum is very small, so the
incident light must be highly monochromatic.
Self-test 12.5 Repeat the calculation for the rotational Raman spectrum of NH3
( = 9.977 cm1).
[Stokes lines at 29 637.3, 29 597.4, 29 557.5, 29 517.6 cm1,
anti-Stokes lines at 29 757.1, 29 797.0 cm1]
12.7 Nuclear statistics and rotational states
Key point The appearance of rotational spectra is affected by nuclear statistics, the selective occupation of rotational states that stems from the Pauli principle.
If eqn 12.25 is used in conjunction with the rotational Raman spectrum of CO2, the
rotational constant is inconsistent with other measurements of CO bond lengths. The
results are consistent only if it is supposed that the molecule can exist in states with
even values of J, so the Stokes lines are 2 0, 4 2, . . . and not 5 3, 3 1, . . . .
12.7 NUCLEAR STATISTICS AND ROTATIONAL STATES
The explanation of the missing lines is the Pauli principle and the fact that 16O
nuclei are spin-0 bosons: just as the Pauli principle excludes certain electronic states,
so too does it exclude certain molecular rotational states. The form of the Pauli principle given in Section 9.4b states that, when two identical bosons are exchanged, the
overall wavefunction must remain unchanged in every respect, including sign. When
a CO2 molecule rotates through 180, two identical O nuclei are interchanged, so the
overall wavefunction of the molecule must remain unchanged. However, inspection
of the form of the rotational wavefunctions (which have the same form as the s, p, etc.
orbitals of atoms) shows that they change sign by (1) J under such a rotation
(Fig. 12.19). Therefore, only even values of J are permissible for CO2, and hence the
Raman spectrum shows only alternate lines.
The selective occupation of rotational states that stems from the Pauli principle is
termed nuclear statistics. Nuclear statistics must be taken into account whenever a
rotation interchanges equivalent nuclei. However, the consequences are not always as
simple as for CO2 because there are complicating features when the nuclei have
nonzero spin: there may be several different relative nuclear spin orientations consistent with even values of J and a different number of spin orientations consistent with
odd values of J. For molecular hydrogen and uorine, for instance, with their two
identical spin- 12 nuclei, we show in the following Justication that there are three times
as many ways of achieving a state with odd J than with even J, and there is a corresponding 3:1 alternation in intensity in their rotational Raman spectra (Fig. 12.20). In
general, for a homonuclear diatomic molecule with nuclei of spin I, the numbers of
ways of achieving states of odd and even J are in the ratio
Number of ways of achieving odd J 1 (I + 1)/I for half-integral spin nuclei
=2
Number of ways of achieving even J 3 I/(I + 1) for integral spin nuclei
461
J=2
+
J=1
+
J=0
Fig. 12.19 The symmetries of rotational
wavefunctions (shown here, for simplicity
as a two-dimensional rotor) under a
rotation through 180. Wavefunctions with
J even do not change sign; those with J odd
do change sign.
(12.26)
For hydrogen, I = 12 , and the ratio is 3:1. For N2, with I = 1, the ratio is 1:2.
Justication 12.1 The effect of nuclear statistics on rotational spectra
Hydrogen nuclei are fermions, so the Pauli principle requires the overall wavefunction to change sign under particle interchange. However, the rotation of an H2
molecule through 180 has a more complicated effect than merely relabelling the
nuclei, because it interchanges their spin states too if the nuclear spins are paired
(; Itotal = 0) but not if they are parallel (, Itotal = 1).
First, consider the case when the spins are parallel and their state is (A)(B),
(A)(B) + (B)(A), or (A)(B). The (A)(B) and (A)(B) combinations
are unchanged when the molecule rotates through 180 so the rotational wavefunction must change sign to achieve an overall change of sign. Hence, only odd values
of J are allowed. Although at rst sight the spins must be interchanged in the combination (A)(B) + (B)(A) so as to achieve a simple A B interchange of labels
(Fig. 12.21), (A)(B) + (B)(A) is the same as (A)(B) + (B)(A) apart from
the order of terms, so only odd values of J are allowed for it too. In contrast,
if the nuclear spins are paired, their wavefunction is (A)(B) (B)(A). This
combination changes sign when and are exchanged (in order to achieve
a simple A B interchange overall). Therefore, for the overall wavefunction to
change sign in this case requires the rotational wavefunction not to change sign.
Hence, only even values of J are allowed if the nuclear spins are paired. In accord
with the prediction of eqn 12.26, there are three ways of achieving odd J but only one
of achieving even J.
Frequency
Fig. 12.20 The rotational Raman spectrum
of a diatomic molecule with two identical
spin- 12 nuclei shows an alternation in
intensity as a result of nuclear statistics.
The Rayleigh line is much stronger than
depicted in the gure; it is shown as a
weaker line to improve visualization of
the Raman lines.
12 MOLECULAR SPECTROSCOPY 1: ROTATIONAL AND VIBRATIONAL SPECTRA
Rotate
by 180
(1)
Change sign
462
Change
sign if
antiparallel
Fig. 12.21 The interchange of two identical
fermion nuclei results in the change in sign
of the overall wavefunction. The relabelling
can be thought of as occurring in two steps:
the rst is a rotation of the molecule; the
second is the interchange of unlike spins
(represented by the different colours of the
nuclei). The wavefunction changes sign in
the second step if the nuclei have
antiparallel spins.
Lowest rotational state
of ortho-hydrogen
Different relative nuclear spin orientations change into one another only very
slowly, so an H2 molecule with parallel nuclear spins remains distinct from one with
paired nuclear spins for long periods. The two forms of hydrogen can be separated by
physical techniques, and stored. The form with parallel nuclear spins is called orthohydrogen and the form with paired nuclear spins is called para-hydrogen. Because
ortho-hydrogen cannot exist in a state with J = 0, it continues to rotate at very low
temperatures and has an effective rotational zero-point energy (Fig. 12.22). This
energy is of some concern to manufacturers of liquid hydrogen, for the slow conversion of ortho-hydrogen into para-hydrogen (which can exist with J = 0) as nuclear
spins slowly realign releases rotational energy, which vaporizes the liquid. Techniques
are used to accelerate the conversion of ortho-hydrogen to para-hydrogen to avoid
this problem. One such technique is to pass hydrogen over a metal surface: the
molecules adsorb on the surface as atoms, which then recombine in the lower energy
para-hydrogen form.
The vibrations of diatomic molecules
In this section, we adopt the same strategy of nding expressions for the energy levels,
establishing the selection rules, and then discussing the form of the spectrum. We
shall also see how the simultaneous excitation of rotation modies the appearance of
a vibrational spectrum.
12.8 Molecular vibrations
Key point The vibrational energy levels of a diatomic molecule modelled as a harmonic oscillator
depend on a force constant k f (a measure of the bonds stiffness) and the molecules effective mass.
We base our discussion on Fig. 12.23, which shows a typical potential energy curve (as
in Fig. 10.1) of a diatomic molecule. In regions close to Re (at the minimum of the
curve) the potential energy can be approximated by a parabola, so we can write
V = 12 kf x 2
J=1
Thermal
relaxation
J=0
Lowest rotational state
of para-hydrogen
Fig. 12.22 When hydrogen is cooled, the
molecules with parallel nuclear spins
accumulate in their lowest available
rotational state, the one with J = 1.
They can enter the lowest rotational state
(J = 0) only if the spins change their relative
orientation and become antiparallel.
This is a slow process under normal
circumstances, so energy is slowly released.
x = R Re
Parabolic
potential energy
(12.27)
where kf is the force constant of the bond. The steeper the walls of the potential (the
stiffer the bond), the greater the force constant.
To see the connection between the shape of the molecular potential energy curve
and the value of kf , note that we can expand the potential energy around its minimum
by using a Taylor series, which is a common way of expressing how a function varies
near a selected point (in this case, the minimum of the curve at x = 0):
A d2V D
A dV D
E x + 12 B 2 E x 2 +
V(x) = V(0) + B
C dx F 0
C dx F 0
(12.28)
The notation (. . .)0 means that the derivatives are rst evaluated and then x is set
equal to 0. The term V(0) can be set arbitrarily to zero. The rst derivative of V is
zero at the minimum. Therefore, the rst surviving term is proportional to the square
of the displacement. For small displacements we can ignore all the higher terms, and
so write
A d2V D
V(x) 12 B 2 E x 2
C dx F 0
(12.29)
You might also like
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2No ratings yet
- 3 Rotational Spectroscopy1Document19 pages3 Rotational Spectroscopy1Omprakash LatiyalNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Spectroscopy TutorialDocument12 pagesSpectroscopy TutorialPratyush RanjanNo ratings yet
- Spectroscopy - Rotational Spectroscopy - WikiversityDocument36 pagesSpectroscopy - Rotational Spectroscopy - WikiversityKishore KishoreNo ratings yet
- Spectroscopy - Rotational Spectroscopy - Wikiversity PDFDocument36 pagesSpectroscopy - Rotational Spectroscopy - Wikiversity PDFKishore KishoreNo ratings yet
- Spectroscopy - Rotational Spectroscopy - WikiversityDocument9 pagesSpectroscopy - Rotational Spectroscopy - WikiversityDr. Gazi Jahirul ISlamNo ratings yet
- Rotational Spectroscopy Part 1Document17 pagesRotational Spectroscopy Part 1shrivastavashubhang02No ratings yet
- Spectroscopy Rotational SpectrosDocument7 pagesSpectroscopy Rotational SpectrosMONERAH D ALOSAIMINo ratings yet
- Photochemistry: Prof. M.N.R. Ashfold (S305)Document66 pagesPhotochemistry: Prof. M.N.R. Ashfold (S305)betjodaNo ratings yet
- Microwave Spectroscopy-1Document35 pagesMicrowave Spectroscopy-1surajjamal111No ratings yet
- Student Solutions ch10 PDFDocument21 pagesStudent Solutions ch10 PDFSergio Magalhaes FerreiraNo ratings yet
- Rigid RotorDocument15 pagesRigid Rotormeghna100% (1)
- First Week-Rovibrational Spectra - End Topic: Rovibrational SpectraDocument4 pagesFirst Week-Rovibrational Spectra - End Topic: Rovibrational SpectraZtt ZhangNo ratings yet
- Angular Dependence of The Sputtering Yield From A Cilindrical TrackDocument6 pagesAngular Dependence of The Sputtering Yield From A Cilindrical TrackCatalinaNo ratings yet
- Microwave SpectrosDocument46 pagesMicrowave SpectrosMOHAMMED ABDUL HAINo ratings yet
- NMR MS Spectroscopy Ambo2 BDocument145 pagesNMR MS Spectroscopy Ambo2 Bkiya01No ratings yet
- Introduction To Molecular SpectrosDocument13 pagesIntroduction To Molecular Spectrosapi-250366166No ratings yet
- Atomic and Molecular Spectroscopy Lecture 2Document29 pagesAtomic and Molecular Spectroscopy Lecture 2Hammed LawalNo ratings yet
- Notes SpectraDocument8 pagesNotes SpectraSUDIPTA SADHUKHANNo ratings yet
- Random Physics ArticlesDocument44 pagesRandom Physics ArticlesAzam-Savaşçı Anderson MohammadNo ratings yet
- Rotational and Vibrational SpectrosDocument11 pagesRotational and Vibrational SpectroswarriorsNo ratings yet
- Vibration-Rotation Spectrum of Co: Rice University Physics 332Document10 pagesVibration-Rotation Spectrum of Co: Rice University Physics 332Bishnu RakshitNo ratings yet
- AstaInfrared SpectrosDocument14 pagesAstaInfrared SpectrosEub EuNo ratings yet
- Electronic Absorption Spectroscopy: M, E, A ADocument8 pagesElectronic Absorption Spectroscopy: M, E, A ANiko DidicNo ratings yet
- Vibration and Rotation SpectrosDocument6 pagesVibration and Rotation SpectrosWendyLimNo ratings yet
- Report On Raman ScatteringDocument7 pagesReport On Raman ScatteringsilentShoeNo ratings yet
- Atkin 12001 Molekular Spectroscopy 001Document5 pagesAtkin 12001 Molekular Spectroscopy 001Nikke ArdilahNo ratings yet
- Cyclotron Training - Part 1 - BasicsDocument31 pagesCyclotron Training - Part 1 - BasicsHariNo ratings yet
- Orbit DecayDocument5 pagesOrbit DecayJames MorseNo ratings yet
- APhO 2018Document88 pagesAPhO 2018Aditya MondalNo ratings yet
- Теоретический тур PDFDocument18 pagesТеоретический тур PDFVokerleeNo ratings yet
- The Essential Components of A Communication System Are The TransmitterDocument5 pagesThe Essential Components of A Communication System Are The TransmitterjnvchindwaraonlineexamNo ratings yet
- Circular Dicroism (CD) and Optical Rotational Dispersion (ORD)Document25 pagesCircular Dicroism (CD) and Optical Rotational Dispersion (ORD)Ananta TadeNo ratings yet
- Microwave SpectrosDocument46 pagesMicrowave Spectros5fdt78kgscNo ratings yet
- Microwave Infrared: SpectrosDocument66 pagesMicrowave Infrared: SpectrosPrathamesh Dash100% (2)
- Xia 2018Document7 pagesXia 2018yaxuanzh321No ratings yet
- Energy States of MoleculesDocument12 pagesEnergy States of MoleculesBenjamín Marc Ridgway de SassouNo ratings yet
- Zeeman EffectDocument5 pagesZeeman EffectTay Xiu WenNo ratings yet
- Class Xii Full Mock 2Document3 pagesClass Xii Full Mock 2physicshari.bwnNo ratings yet
- Dipole Dipole Interactions and FRETDocument12 pagesDipole Dipole Interactions and FRETMartín FigueroaNo ratings yet
- Chemistry 1A Spectroscopy.: Prof. Mike Ashfold (S305) (Mike - Ashfold@bris - Ac.uk)Document63 pagesChemistry 1A Spectroscopy.: Prof. Mike Ashfold (S305) (Mike - Ashfold@bris - Ac.uk)Abd El-Fattah Mohamed OufNo ratings yet
- Wireless Power Transfer Via Strongly Coupled Magnetic ResonancesDocument11 pagesWireless Power Transfer Via Strongly Coupled Magnetic Resonancesapi-3805241100% (1)
- Circular WaveguideDocument19 pagesCircular WaveguideLam DinhNo ratings yet
- LCR CircuitDocument14 pagesLCR CircuitCOSM-OS50% (2)
- Solution To Question Paper IV, V.U. 2013Document9 pagesSolution To Question Paper IV, V.U. 2013Abhijit Kar GuptaNo ratings yet
- Experiement E1 Lab ReportDocument4 pagesExperiement E1 Lab ReportrachelstirlingNo ratings yet
- S-2 Investigating The Fine Structure of H and D Using Fabry-Perot InterferometryDocument5 pagesS-2 Investigating The Fine Structure of H and D Using Fabry-Perot InterferometryAustin Yu LiuNo ratings yet
- Electric Properties of Material II: The Frequency Dependence of Polarization P P P PDocument9 pagesElectric Properties of Material II: The Frequency Dependence of Polarization P P P PPhani KrishnaNo ratings yet
- Chapter 8Document9 pagesChapter 8siddpawar08No ratings yet
- Classical Theory of Rayleigh and Raman Scattering 18pDocument18 pagesClassical Theory of Rayleigh and Raman Scattering 18psatishmaanNo ratings yet
- Module 3: Molecular Spectroscopy Lecture 12: Electronic SpectrosDocument9 pagesModule 3: Molecular Spectroscopy Lecture 12: Electronic SpectrosAnmol SahuNo ratings yet
- Circular Dichroism: Circular Dichroism (CD) Refers To The Differential Absorption of Left and RightDocument21 pagesCircular Dichroism: Circular Dichroism (CD) Refers To The Differential Absorption of Left and RightPeeyush SrivastavaNo ratings yet
- Letters of J.: Physical Review SizeDocument4 pagesLetters of J.: Physical Review SizeDebayan DasguptaNo ratings yet
- Beamdistotion GapDocument3 pagesBeamdistotion GaprajarpitNo ratings yet
- Impedance, Angular Velocities & Frequencies of Oscillating CurrentsDocument32 pagesImpedance, Angular Velocities & Frequencies of Oscillating CurrentselectrosciNo ratings yet
- Optical Rotatory Dispersion Is The Variation in The: Optical Rotation Wavelength Light Absolute Configuration SucroseDocument6 pagesOptical Rotatory Dispersion Is The Variation in The: Optical Rotation Wavelength Light Absolute Configuration Sucrosedhungelsubhash8154No ratings yet
- 1454924410CHE P8 M24 E-TextDocument8 pages1454924410CHE P8 M24 E-TextVimalNo ratings yet
- IsotropicDocument7 pagesIsotropicSiddiqui M. M.No ratings yet
- Experimental Techniques and Synthesis MethodDocument27 pagesExperimental Techniques and Synthesis MethodMónica LedesmaNo ratings yet
- Molecular Orbital Theory (MOT) : Student Worksheet of Physical Chemistry I: Chemical Structure & BondingDocument5 pagesMolecular Orbital Theory (MOT) : Student Worksheet of Physical Chemistry I: Chemical Structure & BondingNikke ArdilahNo ratings yet
- Agend A: Your Text HereDocument26 pagesAgend A: Your Text HereNikke ArdilahNo ratings yet
- Hydrogen and Many-Electron AtomsDocument4 pagesHydrogen and Many-Electron AtomsNikke ArdilahNo ratings yet
- Lampiran PerhitunganDocument2 pagesLampiran PerhitunganNikke ArdilahNo ratings yet
- Lampiran PerhitunganDocument4 pagesLampiran PerhitunganNikke ArdilahNo ratings yet
- Atkin 12001 Molekular Spectroscopy 001Document5 pagesAtkin 12001 Molekular Spectroscopy 001Nikke ArdilahNo ratings yet
- Atkin 10001 VBT 001Document1 pageAtkin 10001 VBT 001Nikke ArdilahNo ratings yet
- Atkin 12001 Molekular Spectroscopy 003Document5 pagesAtkin 12001 Molekular Spectroscopy 003Nikke ArdilahNo ratings yet
- Atkin 10001 VBT 003Document1 pageAtkin 10001 VBT 003Nikke ArdilahNo ratings yet
- My Family N BINDocument17 pagesMy Family N BINNikke ArdilahNo ratings yet
- Ms. Decker's Third-Grade Class: Welcome, Students!Document17 pagesMs. Decker's Third-Grade Class: Welcome, Students!Nikke ArdilahNo ratings yet
- Edi Kurniawan PKA 2010 103194067Document2 pagesEdi Kurniawan PKA 2010 103194067Nikke ArdilahNo ratings yet
- Santos FC Managed To Hold On To Their Crown Jewelry Because The Brazilian Club President atDocument3 pagesSantos FC Managed To Hold On To Their Crown Jewelry Because The Brazilian Club President atNikke ArdilahNo ratings yet
- B.inggris DitaDocument2 pagesB.inggris DitaNikke ArdilahNo ratings yet
- 110KV Cable Termination StructureDocument1 page110KV Cable Termination Structureisan.structural TjsvgalavanNo ratings yet
- Macro Photography Cheat SheetDocument1 pageMacro Photography Cheat SheetRhiannon MorseNo ratings yet
- Waves & Wave Optics - Rudra SeriesDocument182 pagesWaves & Wave Optics - Rudra SeriesParam shahNo ratings yet
- Design of Microwave Wideband Antenna For Brain Tumor Imaging ApplicationsDocument5 pagesDesign of Microwave Wideband Antenna For Brain Tumor Imaging ApplicationsRodina RagabNo ratings yet
- Week 3 SID OID 122Document16 pagesWeek 3 SID OID 122tranlehuyNo ratings yet
- Week 1 Lab #2 - Microscopy & Microscopic Examination of Living MicroorganismsDocument53 pagesWeek 1 Lab #2 - Microscopy & Microscopic Examination of Living MicroorganismsNgoc PhamNo ratings yet
- Microwave Technology Advanced BakingDocument39 pagesMicrowave Technology Advanced BakingEdilyn CalditoNo ratings yet
- Advanced Level Physics Past Papers Index StudentsDocument9 pagesAdvanced Level Physics Past Papers Index Studentstaryll_01No ratings yet
- Phy 102 4 Physics 4 2 2Document4 pagesPhy 102 4 Physics 4 2 2Sagar AcharyaNo ratings yet
- Fundamentals of Ultrasound ImagingDocument11 pagesFundamentals of Ultrasound ImagingAryan Shukla100% (1)
- Structure of Atom MAster Explanation PRESS4Document105 pagesStructure of Atom MAster Explanation PRESS4Chad Ashley VandenbergNo ratings yet
- Kaishengfeng 60825Document11 pagesKaishengfeng 60825Marcio souzaNo ratings yet
- Dark EarthDocument90 pagesDark EarthJay Alfred100% (2)
- PuzzlesDocument4 pagesPuzzlessumasivaraviNo ratings yet
- Analysis of Power Received W.R.T Distance For Different Path Loss ExponentsDocument4 pagesAnalysis of Power Received W.R.T Distance For Different Path Loss ExponentshiNo ratings yet
- Mind MapsDocument3 pagesMind MapsGiannaNo ratings yet
- Enhancement Performance Tapered Slot Vivaldi Antenna For Weather Radar ApplicationDocument4 pagesEnhancement Performance Tapered Slot Vivaldi Antenna For Weather Radar ApplicationayhaynglawuNo ratings yet
- E F G H: (18) RadioactivityDocument5 pagesE F G H: (18) RadioactivityJustin BirdNo ratings yet
- Optoelectronics Devices and SensorsDocument30 pagesOptoelectronics Devices and SensorsAngelica50% (2)
- 06-02-2022 SR - Iit Star Batch II Jee-Adv 2011-p1 Pta-14 Q.P FinalDocument11 pages06-02-2022 SR - Iit Star Batch II Jee-Adv 2011-p1 Pta-14 Q.P FinalCE-026 BharadwajaNo ratings yet
- KCET2022 Physics: Chapter Wise Marks DistributionDocument1 pageKCET2022 Physics: Chapter Wise Marks DistributionG GnaneshwarNo ratings yet
- ILN Pro Additional AnalysisDocument4 pagesILN Pro Additional Analysismaya22scottNo ratings yet
- Physical Sciences p1 Memo - GR 10 - March 2023 (20!03!2023) - 2Document4 pagesPhysical Sciences p1 Memo - GR 10 - March 2023 (20!03!2023) - 2Sherissa HiralallNo ratings yet
- Asme V Art 9Document3 pagesAsme V Art 9ndt_inspectorNo ratings yet
- TOFD - CalculatorDocument9 pagesTOFD - CalculatorDaniel Jimenez MerayoNo ratings yet
- Calibrating Eyepiece Graticule Using Micrometer / Calculating Linear MagnificationDocument17 pagesCalibrating Eyepiece Graticule Using Micrometer / Calculating Linear MagnificationAida MalikovaNo ratings yet
- ITT American Electric Ultra Flood Series 277 & 278 Spec Sheet 2-80Document6 pagesITT American Electric Ultra Flood Series 277 & 278 Spec Sheet 2-80Alan MastersNo ratings yet
- Signalers and Receivers Mechanisms and Evolution of Arthropod CommunicationDocument427 pagesSignalers and Receivers Mechanisms and Evolution of Arthropod CommunicationThet TunNo ratings yet
- Prize-Winning Cameras For Prize-Winning PicturesDocument34 pagesPrize-Winning Cameras For Prize-Winning PicturesDocNo ratings yet
- Translation Product Spec Sheets v1 Sept 2023Document4 pagesTranslation Product Spec Sheets v1 Sept 2023Pepe LolNo ratings yet