Professional Documents
Culture Documents
Denture Base Resin Cytotoxity
Uploaded by
jinny1_0Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Denture Base Resin Cytotoxity
Uploaded by
jinny1_0Copyright:
Available Formats
CarulA.
Lefehvre, DDS, MS'
George S. Schuster, DDS. MS. PhD'
lohn C.
Marr"*
Kent L. Knoernscbild, DDS.
The Effect of pH on the
Cytotoxicity of Eluates
From Denture Base Resins
MS""
School of Dentistry
Medical College of Georgia
Augusts, Georgia
This in uitro study examined the effects of environmental pH on eiution of
potentially toxic substances from heat-, light-, and dual- (chemical plus light)
polymerized denture base resins. Eluates were prepared by daily transfer of
disks to fresh huffets at pH 4.0, 5,0, and 6,8 over a 5-day period. Oral
epithelial cells were plated in culture dishes in medium containing the
eluate. After 24 hours, cellular RNA synthesis was assessed by measuring
tritiated uridine uptake. Effects of materials were compared to identical
cultures that contained the appropriate buffer without the eluate. The results
indicate that the cytotoxic components leach out of the denture base resins
in different amounts and at different rates, and the amount of leaching can be
affected by pH. Int I Prostitodont
1995;a:U2-126.
monomer content of resins polymerized by a long
cycle (greater than 6 hours).'" To minimize the
amount of residual monomer released from the
denture following completion of polymerization,
several authors have suggested that prostheses
should be soaked in water prior to placement.-'^"
Ruyter' demonstrated release of formaldehyde from
denture base polymers and Oysaed et a l " showed
that this compound was still detectable from "dental composites" after 115 days.
Visible light-polymerized denture base resins
have a variety of applications. Advantages of these
resins in the fabrication of complete and removable partial dentures include the absence of free
methyl methacrylate, low toxicity, as well as the
ability to be partially polymerized in the mouth or
on a cast," '" Although one light-polymerized denture baso resin was initially reported to be nontoxic
Msc/ae Praesor, Section ol Removable Prosliiodonlic'^,
after polymerization," subsequent studies have
Department of Oral Rehabilitation,
shown that this resin, which only uses visible light
"lone and Arthur Merrill Professor, Departmenti ol Oral
as its polymerization activator, and two other
Biology & Oral Rehabilitation.
resins, using both light and chemical activation for
'*'Dental Studenl.
p o l y m e r i z a t i o n , have varying levels of
""Aisociale Professor. Section of Removable Proslhodontia,
Deparirrienl of Orai Rhabilitation.
cytotoxicity.'" Although some of these resins contain no methyl methacrylate, they do contain BisReprint requests: Dr Carol A. Lefebvre, Section ol Removable
CMA. Hanks et al'"' showed Bis-GMA to be toxic Jn
Proi!hodontic5, Depailment of Oral Rehabilitation, Sciiool of
two cell culture systems. This toxicity was postuDentisiry. Medical Coliege ol Georgia, Augusta. Georgia
lated to be the result of either new toxic pioducts
30912-1250.
variety of potentially toxic substances have
been shown to elute from auto- and heatpolymerized denture base resins. These include
formaldehyde, methyl methacrylate, methacrylic
acid, and benzoic acid.' ' In addition, the liquid
monomer contained in these resins is an allergen,
and presence of residual monomer in prostheses
has been reported to cause reactions on the skin
and oral mucosa,'"'* The amount of residual
monomer present is dependent upon the type of
denture base resin, the type of polymerization
reaction, the duration of the polymerization cycle,
and the thickness of the resin. Moreover, it has
been shown that denture base resins produced
using a short polymerization cycle (less than 2
hours) contained up to seven times the residual
I o Pro5tliodnrtii
122
pH tderr on Cylotoiicity ol Ocnlure Base Hesins
that arise upon polymerization, or residual toxic
products from an incomplete reaction, Light-poiymerized resins do contain potentially cytotoxic
substances. Using gas chromatography, Tanaka et
al identified residual TECDMA and Bis-GMA in
visible light-polymerized denial resin composite.
Residual Bis-GMA monomer in dental resin composites has been reported to be 0.4% to 1,21 % of
the original weight,-"
Lefebvre et at" studied the length of time and
pattern of release of cytotoxic substances from four
light-polymerized denture base resins. They found
that multiple components leach out of these materials and have cytotoxic effects on oral epithelial
cells. The different levels of toxicity among the various materials suggest that different components
may be leaching out at different rates, and that the
release of cytotoxic resin components may continue for several days. Furthermore, the above
study demonstrated that because the toxic components are diffusible in an aqueous environment,
they may be capable of affecting tissue sites distant
from the resin contact area. This may be a particular problem for patients having mucosa that is
infected, inflamed, lacerated, or fragile as a result
of nutritional problems or concurrent medications.
Thus, large areas of the oral mucosa may be
exposed to these toxic components over an
extended period of time.
Most Studies have been conducted in a neutral
environment (pH 7,0 to 7.4), However, the natural
medium to w h i c h these resins are exposed
includes a wide variety of foods and liquids of varied composition and, of course, saliva, all of which
cause the environment to fluctuate over a relatively
great range ot" physcochemical conditions. One of
the more obvious variables is pH. Koda et al'
demonstrated differences in leachability of denture
base acrylic resins at a range of pH from 4.0 to 6,8,
Whether a change in pH could cause a change in
the quantity or quality of toxic components eluted
from visible light-polymerized denture base resins
has not been fully examined. The purpose of this
study was to examine the effects of pH on the biocompatibility of visible light-polymerized denture
base resins compared to a widely used heat-polymerized resin.
national, York, PA); Triad (Denfsply International);
and Fxtoral (ProDen Systems, Portland, OR),
Lucitone 199 is a high-impact, heat-polymerized
denture base resin. Triad is a one-component
paste, containing light-polymerizing urethane
dimethacrylate. Fxtora) is a co-polymer of
polyiethyl m etb acryl a te) and poly(methyl
methacrylate) and is a dual- (light and chemical)
polymerized resin,'- The formulations of the lightpolymerized denture ba.se resins marketed as denture reline materials were used.
Sample Fabrication
Triplicate sample disks of the denture base resins
were fabricated under aseptic conditions in molds
1 cm in diameter by 1 mm thick. This sample configuration was selected because it is the minimum
thickness that would be present in a complete or
removable partial denture, would allow complete
polymerization, and fit the experimental system by
allowing tbe medium to completely cover the samples. Samples were fabricated according to the
manufacturers' directions to produce two types of
samples: (1) samples processed without the air barrier coatings (ABC), and (2) samples processed with
the air barrier coatings placed during processing,
removed (as recommended by the manufacturer)
with a sterile brush and water, and rinsed in sterile
water for 30 seconds. The air barrier coatings used
were those provided by the manufacturers for their
respective materials. Ali materials were polymerized according to manufacturers' directions using
the Triad II Light (Dentsply International).
Eluate Preparation
Michaelis' barbital buffer'^ (0,1 M) was prepared at
pH 4,0, 5,0, or 6,8, This buffer was selected because
it provided buffering at all three pH levels. Four resin
discs were placed in sterile glass vials containing 3
mL of buffer. The vials were placed on a rocking
table (100 rpm) for 24 hours at room temperature.
The disks were transferred aseptically lo new vials
daily for 5 days, and the process was repeated.
Dulbecco's Minimum Essential Medium (DMEM)
IGibco, Grand Island, NY) supplemented with 5%
fetal bovine serum (FBS) (Gibco), 100 U/mL penicillin, and 100 pg/mL streptomycin was added to
each of the three buffers to bring them to the physiologic pH range. The medium was originally buffered
to pH 7.8. The Michaelis buffer was diluted 1 ;4 with
medium that was concentrated by 25% so that the
final osmolarit\' was normal for fhe medium, and the
eluate-containing buffer-medium solution so pro-
Materials and Methods
Denture Base Resins
The materials selected are representative of different types of polymerization reactions. The materials tested were Lucitone 199 (Dentsply Inter-
), Number?, 1995
123
The Inlernational lour nal of Proslhodonlics
pH Effeit on Cylotoxititv of Denture Base Resii
ining the interactions. Data were converted to percent control to facilitate comparison. Since tbe
buffer itself produced a slight inhibition of growtb at
all pHs, the cells in medium containing appropriate
buffer but no eluate were used as controls.
duced was twice the concentration of that from previous, comparable studies,"-''' The final pH for the
media containing eluates in the pH 4,0, 5,0, and
6,8 buffers was pH 7,2, 7.6, and 7,7, respectively.
Cell Culture
Results
As previously described,'' an established line of
randomly bred hamster cheek pouch epithelial
cells was used in these experiments. The cultures
were maintained at 37C in an atmosphere of 5%
CO^95% air in DMFM medium containing 5%
FBS and antibiotics.
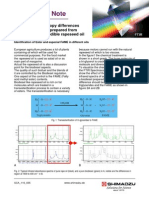
Figures 1 to 3 show the effects of eluates from
the three materials at each pH on RNA synthesis.
Only eluates from the first 3 days are presented.
Thereafter no differences were evident, Elution
from Lucitone 199 exposed to pH 4,0 buffer for 24
hours (day 1) produced a significant inhibition of
RNA synthesis. In eluates from the second day,
RNA synthesis was significantly elevated over control levels, and by day 3, equal to control levels
(Fig 1 ), At pH 5,0 the eluate from day 1 resulted in
some stimulation, while those from days 2 and 3
were not significantly different from control.
Material eluted at pH 6,8 produced no significant
response as compared to control cultures.
Triad (Fig 2) eluted during the first day at all
three pH levels produced significant inhibition of
RNA synthesis as compared to control cultures.
Apparently most of the inhibitory material was
eluted the first day, since by after the second day
of elution, none of the eluates, except that in pH
5,0 buffer, was significantly inhibitory. While the
response to the pH 5,0 eiuate from any day did not
significantly differ from that produced by the other
two pH levels on every day, it was consistently
more inhibitory than the others. This trend suggests
that at pH 5,0 more cytotoxic material may eluate
from this material than at pH 4,0 or 6,8.
Figure 3 shows the results for Fxtoral, This resin
elutes significantly cytotoxic levels of material at
all three pH levels on both days 1 and 2. Again,
while not significantly different from each other,
the trend at day 2 suggests that, at lower pHs, more
cytotoxic material is released. The material
released on the third day of soaking also appeared
to be cytotoxic, but the RNA response was more
variable, as the results were not significantly different from control.
The results of this study using Extoral differed
considerably from those of prior studies.'"" The
material tested in this study was a different lot of
material from that used previously. Since only a
small amount of the prior material remained a
comparative study of the two lots was conducted.
Figure 4 shows the effects of eluates from the two
different lots of Extoral compared to the appropriate control buffer. Lot 1 (authors' designation) is
the material tested in the previous study and Lot 2
Metabolic Assays
Three thousand oral epithelial cells, suspended in
50 |JL of eluate plus 50 [jL of fresh medium, were
added to each well of 96 well culture plates. This
allowed eight samples per material, per test, and
treatment. This final 1:2 dilution in medium
brought the buffer to a final concentration of 0,125
M, and the ratio of disk and eluate:culture medium
was comparable to that of the authors' prior
studies.""'" Plates were placed on a rocking platform for 30 minutes and returned to the incubator
for 24 hours, Weils containing cells in medium
alone and medium containing suitably diluted
buffer in which no resin had been soaked were
used as controls.
After this initial 24-hour period, cellular
metabolism, as reflected by RNA synthesis, was
monitored. Cells were labeled by adding 15
pCi/mL' H-Uridine (Specific Activity equal to 35,8
Ci/mol; New England Nuclear, Boston, MA, After
24 hours of incubation the isotope-containing
media were removed, the cells washed with Trisbuffered saline, pH 7,4, then 100 [jL of 10% (V/V)
trichloroacetic acid (TCA) was put into each well.
After washing an additional three times with TCA,
the samples were solubilized in Laemmelis' solubilization buffer-'' (0,0625moi/L Tris-hydrochloride,
pH 6.8, 2% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 10% glycerol), and duplicate aliquots
were counted in a liquid scintillation counter
(model 3801, Beckman Instruments, Fullerton,
CA), All studies were run twice and results were
based on counts per minute (CPM)/weII,
Statistical analysis o tbe data was performed
using a multifactoriai ANOVA (alpha = 0.05] to
determine differences in cytotoxicity based on material, pH, and time. If significant differences were
found, levels of tbe interaction term were used to
define groups for a one-way ANOVA and Duncan's
multiple range test as a post hoc method for exam-
The Irternalfoiia
I of Prosthodontii
124
Volumes.
Leiebvrf L'I .i
pi I Lictt tin C'vloluiicily o( Denliire Base Resins
Fig 1 Relative response ot
oral epithelial cells to eluates
Irom Lucitone 199 exposed at
three pH levels compared to
control cultures, following labeling ot cellular RNA. The arrowheads indicate a significant difference {P < .05] from control.
Fig 2 Relative response of
oral epithelial ceils to eiuates
from Triad exposed at three pH
levels compared to control cultures, following labeling of cellular RNA. The arrowheads
indicate a significant difference
{P< .05) trom control.
jr
75
1
JL JL
T
R
"lil-j T 1
//.
Day 1
Day 2
1 " " 1 pH 4 0
pH 5.0
Day 3
CUD pH 6.e
Discussion
(authors' designation) is the material mainly tested
in the current study- For day 1, ail materials were
generally inhibitory; however, materials from Lot 2
were significantly inhibitory. By day 2 the eluates
approached control levels, except the Lot 1 eluate
at pH 4.0, which was significantly inhibitory. As
above, the lower pH appeared to elute more cytotoxic material.
Since materials with air barrier coating placed
for polymerization then removed did not significantly differ from those without the coating, only
results from the latter are shown.
3, Number 2, 1995
^ H
I 1
Cells cultured in medium containing Michaelis
buffer grow at a slight but not significantly slower
rate than those in piain medium, suggesting a modest inhibition by the buffer. This buffer was chosen,
however, since it permitted use of the same buffering system at all three of the chosen pH levels.
Responses were compared to cells grown in the
comparable buffer-containing medium. The pH
levels of the final eluate buffer-containing media
very close and well within the normal growth
125
The Inte ma lin nal loumal of Proslliodontii
pH Elieci on Cytoioiicity of Denture Base Ream
Pe
cenl C nlro
Fig 3 Relative response of
oral epithelial cells to eluates
from Extoral exposed at three
pHs compared to control ouitures, following labeimg of oellular RNA. The arrowhead indicates a significant difference (P
c .05) from control.
1
-
25 -
Dayi
TB.H
1
1
"1 pH 4.0-Lot 1
^ H
pH e.B-Lol 1
r^^^^
pH 4.0-Lot 2
Fig 4 Relative response of
oral epilfielial ceils to eiuates
from Iwo lots of Extorai
exposed at pH 4.0 or 6.8 compared to control cultures foilowing labeling of ceilular RNA. Loi
1 indicates Exforal material utilized in pnor studies. Lot 2 indicates Estorai maferial uiized
in the current study. The arrowhead indicates a significant difference P< .05) from control.
1 pH 6.8-Lot 2
Day 2
range of the cells. Overall, the results suggest that
the pH of the environment affects the nature atid/or
amount of cytotoxic substance(s that leach out of
resins into the environment. The cellular toxicity of
Lucitone 199 exposed to the lower pH (pH 4.0}
may be the result of methyl methacrylate. Koda et
ah found higher levels of this component in artificial saliva at pH 4.0 following incubation of heatand auto-polymerlzed resins. The stimulation i,een
at higher pH levels and on day 2 is likely related to
release of lower (sublethal) levels of cytoloxic substances. The cells are not as seriously impaired and
compensate for damage by increasing various synthetic responses such as RNA synthesis. Such
responses have been demonstrated in previous
studies using these materials."
Conversely, Triad, while releasing cytotoxic
substances at all three pH levels, especially during day 1, appeared to release more at pH 5.0.
Since salivary pH fluctuates, the release of cytotoxic substances at all three pH levels could produce a greater clinical problem than the methyl
methacrylate, which produces its greatest problem at pH 4.0. Therefore, the pH-related elution
will be transitional, as the pH passes through the
critical ranges. However, the greater tbe pH range
for toxic substance release, the greater the potential for clinical problems. The in vitro response
126
pH Effect on Cylotoiciiy of Denture Base Resins
resulting from maintaining the pH at a constant
level for a 24-hour period reflects the maximum
release in that time increment. The fluctuations
that occur in vivo will likely result in elution of a
lesser total amount in any 24-hour period, but the
total length of time over which such elution can
occur will be prolonged. However, given the
other variables in the oral cavity, these phenomena need further examination before such a conclusion can be drawn. The overall cytotoxicity of
Triad was consistent with prior studies.'"'"
Extoral, a dual light- and chemical-polymerized
resin, showed considerable cytotoxicity at all three
pH levels, especially for the first 2 days of elution.
The results of the second 24-hour period of elution
suggest that lower pH produces greater elution.
Also of interest was the difference in cytotoxicity of
this material in the current studies compared to
prior studies in which it showed little cytotoxicity.
However, direct comparison indicates that this difference resulted from different lots of material. It is
likely there were different types or amounts of
cytotoxic impurities or by-products leaching out of
the two different lots or that the manufacturer
changed the formula. While this could have been
due to differences in polymerization, it is unlikely
since direct comparison of the two lots, polymerized at the same time by the same light, showed
considerable differences in cytotoxicity. This
emphasizes the need for some type of testing for
impurities in materials.
The differences in cytotoxicity of resins without the air barrier coating compared to resins
with it applied then removed were not significant. The authors' prior studies"'" showed cytotoxicity of some air barrier coatings, but inconsistent levels of cytotoxicity if this was removed.
The lack of additional cytotoxicity in current
studies with the air barrier coating suggest more
efficient removal in the current studies, and
emphasize the need to follow the manufacturer's
recommendations regarding its c o m p l e t e
removal after polymerization.
Denture surfaces are exposed to a considerable fluctuation in pH and other environmental
conditions that can result in leaching of cytotoxic substances from the material. These substances can produce a variety of clinical problems when they alter the c e l l ' s metabolic
abilities. For example, impairment of RNA synthesis can, in turn, inhibit production of the keratins necessary for mucosal protection. If subjacent fibroblasts are affected, disturbing collagen
formation, a weaker or more permeable mucosa
could result. While this can occur over several
i. Number 2, 1995
days, in some instances even a brief 1- to 2-day
exposure could have lasting effects. For example, it may facilitate the penetration of other toxins or of eluted substances from the materials
themselves, which then act as allergens. Even a
single exposure is adequate to sensitize an individual and lead to hypersensitivity reactions.
These results emphasize the recommendation of
others that prostheses be soaked in water prior
to placement.-"" They also emphasizes the need
for further studies of materials and their specific
mechanisms of cytotoxicity.
Conclusion
This in vitro study examined the effects of environmental pH on elution of potentially toxic substances from heat-, light-, and dual- (chemical
plus light) polymerized denture base resins. Three
reins were evaluated at pH levels of 4.0, 5.0, and
6.8. Within the design of this study it can be concluded that:
1. Components that leach out of denture base
resins can have an adverse effect on oral
epithelial cells,
2. Leaching of such components can occur in different amounts and at different rates, depending
on environmental pH and the material.
3. Since the pH in the oral cavity varies over a
wide range, the pH-related elution profiles can
affect the clinical response to a material.
References
1. Ruyter IE. The release o formaldehyde frorr denture base
polymers. Acta Odontoi Scand 1930;38:17-27.
2. Baker S, Brooks SC, Walker DM. The release of residual
mororyieric methyl methacrylate from acrylic appliances
m the human mouth: An assay for monomer in saliva. J
Dent Res 1936;67:1395-i299.
3. Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N,
Kawano J. High-performance liquid Chromatographie estimation of eluales from denlurc base polymers, | Dent
1989;17:84-89.
4. Koda T, Tiuchiva H, Yamauchi M, Ohtani S, Takagi N,
Kawano |. Leachability of denture base acrylic resins in
artificial saliva. Dent Mater 1990;6:13-16.
5. Tsuchiya H, Ohtani S, Takagi N. Leachability of denture
base polymers in saliva and its inhibition [abstracti, |
Dent Res 1992,71 (special issue):56B.
6. Fletcher AM, Purnaveja A, Amin WM, Ritchie CM, Moradians
S, Dodd AW. The level of residual monomer in self-curing
deniure-base materials. ] Dent Res 1983;62:118-120.
7. Weaver RE, Coebel WM. Reactions to acrylic resin dental
prostheses. J Prosthet Dent 1980:43:138-142.
127
The Inierraiionai lournal of Prosthodontics
pH Eied in CvMoKicily of Demure Base Re
a,
Slurgis TE, Fink )N, Hypersersilivily lo acrylic re^in. |
Prosthel Dent 1 969;22;425-43e,
9. Fisher AA, Allergic seiisitization of (he skin and oral
mucosa to acrylic resin denlure m.ileriak. | Priisthel Dont
1956;6;593-596,
10, Austin AT, Basker RM, Tht level of residual monomer in
acrylic denlure base maierials, liril Denl | 19H;21-2e6.
11, Lefebvre CA, Knoernschild KL, Schuster OS. Cytolosicity
of eluales irom li|>lit-polymeried derlure base resins, |
Prosthet Dent 1994,72:644-650.
12, Oysaet! H, Ruyter IE, Sjovikskleven I I , Release of
formaldehyde in dental cotnposiles. I Denl Res 1980;
67:1289-1294.
1 3, Osle RE, Sorensen SE, Lewis EA. A new visible lighi-curcd
resin system applied to removable proslhodontics. I
Proslliet Dent 198i>;56:497-506.
14,
15,
IB.
Barron D], Schuster CS, CauRhman GI3, Lei^''^'^ ^ _
iocompatibilily of visible ligbt-polymerized denlure Oas
resins. Im | Proslbodont 19g3;6;495-501,
19. Hanks CT, Anderson M, Craig RG, CylotO!<ic e * ^ ^ "
dentai cements on iwo celi culture systems, I Oral Pathol
I98l;l0;10l-n2,
20. Tanaka K, Taira M. Shintani H, Wakasa K, Yamaki M.
Residual monomers (TEGDMA and Bis-GMAl of a set visible-hght cured dental compoiite resin when immersed in
waler. | Oral Rehabil 1991 ;18:353-i62.
21.
inoue K, Hayashi I. Residual monomer (Bis-GMA) of composite resins, | Oral Rehabil I 9B2;9.493-497,
12.
Smith LT, Powers |M. In vilro properties of lighl-poiymerized reline malerials. l n t | Prosthodont 1991;4:445-448.
23,
Michaelis L. Der Acetat-Veronal Pufier. Biochem Z
193I;234:139-14I,
24,
Okita N, Hensten-Pettersen A, In vitro cylotoxicity of tissue conditioners, I Prosthel Dent 1991 ;66:6S6-&59,
25,
FHensten-Pedersen A, Wiclorin L, The cytotoxic effect of denture base polymers. Ada Odontol Scand 1981 ;39;101-106.
2h.
Laemnili UK, Cleavage of structural prolems during the
assembly o the head of bacleriophage T4, Nature
Khan Z, von Fraunhofer |A, Ra^avi R. The Staining characteristics, transverse strenglh, and microhardness of a visible light-cured denture base material. | Prosthot Denl
1987;57:384-386.
Khan Z, Martin |, Collard S, Adhesion characteristics of vliible light-cured dsnture base maie'ial bonded to resilient
lining materials. I Prosthet Dent 1989;62;196-200.
27,
16,
Conrad RS, Reigor MR, Comparison of heat-, self-, and
light-polymerized acrylics labstract]. | Dent Res 1989;6B
(special issue);972.
1 7, Lefebvre CA, Schuster GS, Caughman CB, Caughnian WF.
Effects of denture base resins on oral epithelial cells, Int I
Proslhodontl991;4i371-376,
1970:227:680-687.
Lefebvre CA, Schuster GS, Richardson DW, Barron D|.
The cyiotoxic effects of denture base resin sealants. Inl ]
Proslhodont 1 99Z;5:;58-562.
iitersture Abstract -
Isothermal Anneal Effect on Microcrack Density around Leucite Particles in
Dental Porcelain
Current dental porcelains for metal liondirig rely upon leucite to provide the higti expansion
necessary for thermal compatibility with metal ceramic alloys. While glass-leucite composite
has been successfully used to create porcelains compatible with dental alloys, the approach
has two fundamental problems. First, the volume fraction of leucite is subject to change during
the various heat treatments encountered during faDncation of restorations. Second, the large
mismatch in coefficient of thermal expansion between the leucite and the matnx results in
microscopic cracks around the leucite particles that partially decouple them from the matrix
glass. The purpose of Ihis study was to determine if isothermal heat treatment resulted in an
alteration of the microcrack density in a dental poicelam. Ten specimens of a dental porceiain
that had previously exhibited an increase in thermal expansion as a function of isothermal
heat treatment were prepared and divided into two groups. The experimental group was
heated to 75O'G and held for 16 minutes before cooling to room temperature. The control
group was heated to 75O'C and immediately withdrav/n from the furnace. The mean microcrack densities were determined by quantitative stereology to be 575 cmVcm' 75 cmVcm^ for
the control group and 231 cm'/cm' for the experimental group. The annealed specimens had a
significantly lower microcrack density than the control specimens. The authors concluded that
annealing ot microcracks and the consequent increase in coupling of leucite to the surrounding glass matrix is an additional explanation lor the increases in porcelain thermal expansion observed during multiple firings, post-soldering, and slow cooling.
Mackert JR, Reuggeberg FA, Lockwood PE, Evans AL, Thompson WO, J Dent Res 1394:73(6):
1221-1227. References: 34. Reprints: Dr JR Macken, Jr. Dii/ision of Dentat Materials, School ol Oeritistry,
Medicai College or Georgia. Augusta. Georgia 30912-12S4.flicdarcf. Seals, Jr, DDS, ME, MS,
Deparlmenl ol Proslhooontics, Unversify ol Texas Health Center al San Antonio, San Amonio, Texas
The Iniernarional Journal of ProsthoiJontii
128
Volume 8, N
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- G - TMD-pedo MNGMT PDFDocument6 pagesG - TMD-pedo MNGMT PDFjinny1_0No ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- KurthDocument18 pagesKurthjinny1_0No ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Pulp Therapy-Guideline AapdDocument8 pagesPulp Therapy-Guideline Aapdjinny1_0No ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Groper's ApplianceDocument4 pagesGroper's Appliancejinny1_0No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Oral Mucosal Lesions Associated With The Wearing of Removable DenturesDocument17 pagesOral Mucosal Lesions Associated With The Wearing of Removable DenturesnybabyNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- A Systematic Review of Impression Technique For ConventionalDocument7 pagesA Systematic Review of Impression Technique For ConventionalAmar BhochhibhoyaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- GicDocument5 pagesGicmrnitin82No ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- E Adaproceedings-Child AbuseDocument64 pagesE Adaproceedings-Child Abusejinny1_0No ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- AAPD Policy On - FluorideUseDocument2 pagesAAPD Policy On - FluorideUsejinny1_0No ratings yet
- Mucosa in Complete DenturesDocument4 pagesMucosa in Complete Denturesspu123No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- EEL 7 SpeakingDocument20 pagesEEL 7 SpeakingSathish KumarNo ratings yet
- Die HardnerDocument4 pagesDie Hardnerjinny1_0No ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Assertmodule 1Document9 pagesAssertmodule 1Olivera Mihajlovic TzanetouNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Article-PDF-Amit Kalra Manish Kinra Rafey Fahim-25Document5 pagesArticle-PDF-Amit Kalra Manish Kinra Rafey Fahim-25jinny1_0No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Spacer Design Jips 2005 PDFDocument5 pagesSpacer Design Jips 2005 PDFjinny1_0No ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Afpam47 103v1Document593 pagesAfpam47 103v1warmil100% (1)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- DisinfectionDocument5 pagesDisinfectionjinny1_0No ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- ResinDocument8 pagesResinjinny1_0No ratings yet
- 7Document8 pages7jinny1_0No ratings yet
- GoldsDocument11 pagesGoldsjinny1_0No ratings yet
- Implant AngulationDocument17 pagesImplant Angulationjinny1_0No ratings yet
- Patient-Assessed Security Changes When Replacing Mandibular Complete DenturesDocument7 pagesPatient-Assessed Security Changes When Replacing Mandibular Complete Denturesjinny1_0No ratings yet
- AlginateDocument5 pagesAlginatejinny1_0No ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Avg Vs NormalDocument1 pageAvg Vs Normaljinny1_0No ratings yet
- Stress Fatigue PrinciplesDocument12 pagesStress Fatigue Principlesjinny1_0No ratings yet
- 11Document7 pages11jinny1_0No ratings yet
- Long-Term Cytotoxicity of Dental Casting AlloysDocument8 pagesLong-Term Cytotoxicity of Dental Casting Alloysjinny1_0No ratings yet
- 3Document7 pages3jinny1_0No ratings yet
- The Technically Impossible HolocaustDocument20 pagesThe Technically Impossible HolocaustHuckelberry100% (2)
- Understanding Internal Energy at the Microscopic LevelDocument4 pagesUnderstanding Internal Energy at the Microscopic Levelaknauriyal2013No ratings yet
- Manufacturing Method For CompositesDocument41 pagesManufacturing Method For CompositestpmendozaNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Anse Co. 2023 Catalog Pipe FittingsDocument600 pagesAnse Co. 2023 Catalog Pipe FittingsShakeer PttrNo ratings yet
- PFOA Factsheet (Revised)Document8 pagesPFOA Factsheet (Revised)AngshumanNo ratings yet
- Classification of Steel - Welding and NDTDocument3 pagesClassification of Steel - Welding and NDTAshif Iqubal100% (1)
- Molecules: Blumea Balsamifera-A Phytochemical andDocument25 pagesMolecules: Blumea Balsamifera-A Phytochemical andAlyssa Leah Veloso EvangelistaNo ratings yet
- Backup Rings Respaldo de OringsDocument8 pagesBackup Rings Respaldo de OringsRPINILLA (EICO S.A.)No ratings yet
- IIW - International Institute of WeldingDocument3 pagesIIW - International Institute of WeldingNilesh MistryNo ratings yet
- 4013 Stability TestingDocument5 pages4013 Stability TestingtghonsNo ratings yet
- SemiconDocument9 pagesSemiconRealyn PugayNo ratings yet
- RRLDocument5 pagesRRLSupremo Family100% (1)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Flow Assurance for Offshore Production - FAOPDocument4 pagesFlow Assurance for Offshore Production - FAOPRajarshiPanigrahiNo ratings yet
- JEE Main 2020 Question Paper Solutions 9 January MorningDocument45 pagesJEE Main 2020 Question Paper Solutions 9 January MorningDishant ShahNo ratings yet
- FTIR Analysis of Rapeseed Oil and Biodiesel Methyl EstersDocument2 pagesFTIR Analysis of Rapeseed Oil and Biodiesel Methyl Estersrgx1120% (1)
- Packaged Drinking WaterDocument4 pagesPackaged Drinking WaterSanjay JainNo ratings yet
- Glyphosate Goker MSDS 1Document7 pagesGlyphosate Goker MSDS 1Bima SitorusNo ratings yet
- Assignment 6Document3 pagesAssignment 6Yi Hong LowNo ratings yet
- Ekatalog 2023 Sulsel RajawaliDocument50 pagesEkatalog 2023 Sulsel RajawaliSafria HamzaNo ratings yet
- Metal CastingDocument40 pagesMetal CastingFahmi Sanji AlexanderNo ratings yet
- Lecture - 3 Dosimetric Quantities and Biological EffectsDocument33 pagesLecture - 3 Dosimetric Quantities and Biological Effectsmz2v8rs7srNo ratings yet
- 4.7 Lab - Percentage of Water in PopcornDocument3 pages4.7 Lab - Percentage of Water in PopcornVansh PatelNo ratings yet
- Steel Forgings, Carbon and Alloy, For General Industrial UseDocument10 pagesSteel Forgings, Carbon and Alloy, For General Industrial UseRed RedNo ratings yet
- Gas Sweetening TotalDocument62 pagesGas Sweetening TotalMehdi AlizadNo ratings yet
- Efficient Synthesis of 3-Hydroxy-1,4-Benzodiazepines Oxazepam and Lorazepam by New Acetoxylation Reaction of 3-Position of 1,4-Benzodiazepine Ring - Organic Process Research & DevelopmentDocument12 pagesEfficient Synthesis of 3-Hydroxy-1,4-Benzodiazepines Oxazepam and Lorazepam by New Acetoxylation Reaction of 3-Position of 1,4-Benzodiazepine Ring - Organic Process Research & DevelopmentSimon GeschwindNo ratings yet
- Duct BurnersDocument23 pagesDuct BurnersMartín Diego MastandreaNo ratings yet
- Manufacturing Ammonia Using The Haber Process: BSC IllDocument8 pagesManufacturing Ammonia Using The Haber Process: BSC Illdigukharade9848No ratings yet
- 17-7 PH Stainless SteelDocument2 pages17-7 PH Stainless Steelzain malikNo ratings yet
- ThermodynamicsDocument12 pagesThermodynamicsWasif RazzaqNo ratings yet
- Wa0044 PDFDocument337 pagesWa0044 PDFYamilNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Napoleon's Buttons: 17 Molecules That Changed HistoryFrom EverandNapoleon's Buttons: 17 Molecules That Changed HistoryRating: 4 out of 5 stars4/5 (25)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet