Professional Documents
Culture Documents
Cyclisations Problems 2013
Uploaded by
Ghadeer M HassanCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cyclisations Problems 2013
Uploaded by
Ghadeer M HassanCopyright:
Available Formats
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
HOMEWORK / REVISION / TEST YOURSELF QUESTIONS
A1:- Naming heterocycles Match the following heterocycles with the names given
Ph
N
H
Br
H3C
N
O
CH3
H3C
N
H
H
N
CH3
OMe
NH
Ph
CH3
H
N
Br
CH3
2-bromoindole
5-methoxyisoquinolin-1-one
2-phenyl-3,4-dihydroquinoline
2,7-dimethylquinolin-4-one
3,8-dimethylisoquinoline
4-phenyl-1,2-dihydroquinoline
7-bromo-2,3-dihydroindole
2,7-dimethylquinoline
A2 Name the following heterocycles
Br
H3C
CH3
N
H
CH3
N
H
H
N
MeO
NH
OMe
CH3
CH3
N
H
N
H
N
Ph
OMe
page 1
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
B:- Formulation of heterocyclic products
B1 Formulate the products from the following reactions, all of which are the Doebnervon Miller variation of the Skraup quinoline synthesis. Identify key pre-cyclisation
intermediates in each case.
H
+
H3C
NH2
Br
2
H2N
Ph
CH3
3
CH3
H3C
NH2
O
4
Br
?
Ph
NH2
O
5
NH2
Ph
?
CH3
Et
Et
NH2
O
+
Ph
Br
7
H2N
H3C
?
CH2CH3
page 2
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
B2 Formulate the products from the following reactions, all of which are quinoline,
quinolone or isoquinoline syntheses. Identify key pre-cyclisation intermediates in
each case.
MeO
CH3
NH2
H+
+
H3C
MeO
O
2
H+
+
O2N
H
NH2
1. Et3N
2. POCl3, heat
Cl
3
Cl
NH2
+
H3C
O
4
Cl
1. Et3N
2. POCl3, heat
3. Pd-C, heat (no H2)
NH2
H3C
+
1. AcOH, 40 oC,
with azeotropic
removal of H2O
Ph
OEt
NH2
H3C
+
?
2. Ph2O, 250 oC
1. 150 oC,
2. conc. H2SO4
Ph
OEt
NH2
Br
7
H2N
O
NaOH, 0 oC
H3C
?
Ph
CH3
Br
8
NH2
H
H2SO4, heat
page 3
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
B3 Formulate the products from the following reactions, all of which are Fischer
indole syntheses. In each case identify the enamine intermediate prior to the [3,3]sigmatropic rearrangement step.
O
1
NHNH2
H3C
O
2
NHNH2
H3C
CH3
NHNH2
Br
4
CH3CH2
H2NHN
Br
CH2CH3
+
NHNH2
H3C
CH3
O
NHNH2
+
MeO
O
7
NHNH2
H3C
Ph
CH3
O
8
CH3
NHNH2
O
+
NHNH2
page 4
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
B4 From what starting materials could you make these two indoles? In these
examples, could there be any problems with formation of isomeric indole products? If
so what is (are) their structure(s)?
1
H3C
?
CH3
N
H
?
MeO
N
H
.
C:- Effect of substituents on reaction rate
For each of the following pairs of reactions, which will have the faster rate of reaction
and why?
C1
O
MeO
MeO
H
+
reaction A
NH2
CH3
CH3
CH3
CH3
CH3
O
O2N
O2N
H
+
NH2
CH3
reaction B
C2
O
MeO
MeO
H
+
reaction A
NH2
CH3
O
H
+
MeO
NH2
CH3
MeO
reaction B
C3
MeO
MeO
NC
NH2
NC
NC
NC
MeO
MeO
CH2O
NC
NH
NC
reaction B
CH2O
NH2
reaction A
MeO
page 5
MeO
NH
HETEROCYCLIC CHEMISTRY Part 2
Modules 06710-12-14
D:- Electrophilic and nucleophilic reactions of heterocycles
Cl
1
Cl
heat
HN
+
N
(1 mole equivalent)
Cl
heat
Na O
(1 mole equivalent)
Cl
Cl
heat
+
N
H2N
Cl
(1 mole equivalent)
CH3
4
heat
HNO3 / H2SO4
Br2
Br2
CH3
N
H
CH3
6
N
H
O
7
N
H
H+ cat
H
O
8
N
H
Br
?
C13H17NO
H+ cat
H
N
H
NaOEt
+
EtO2C
?
heat
Cl
9
CH3I
CO2Et
?
N
Cl
N
H
NH2
page 6
You might also like
- Adrenocorticoids2- ادوية نظري - د.احسانDocument4 pagesAdrenocorticoids2- ادوية نظري - د.احسانGhadeer M HassanNo ratings yet
- Acs سريرية نظري- د.حيدرDocument56 pagesAcs سريرية نظري- د.حيدرGhadeer M HassanNo ratings yet
- Year 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestDocument5 pagesYear 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestGhadeer M HassanNo ratings yet
- 2004 OrgSeminarTestAnswerDocument2 pages2004 OrgSeminarTestAnswerGhadeer M HassanNo ratings yet
- Cycloadditions Problems 2013Document8 pagesCycloadditions Problems 2013Ghadeer M HassanNo ratings yet
- Indole and Benzimidiazole NucleusDocument32 pagesIndole and Benzimidiazole NucleusSrikanth MalipatelNo ratings yet
- Chapter 12 - Heterocyclic CompoundsDocument15 pagesChapter 12 - Heterocyclic CompoundsGhadeer M HassanNo ratings yet
- PK Modeling Approaches for Drug DistributionDocument67 pagesPK Modeling Approaches for Drug DistributionGhadeer M HassanNo ratings yet
- Write Your Answers On This Sheet: N O O O O O PHDocument2 pagesWrite Your Answers On This Sheet: N O O O O O PHGhadeer M HassanNo ratings yet
- Quinoline SynthesisDocument6 pagesQuinoline SynthesisFrancesco TutinoNo ratings yet
- Cycloadditions Solutions 2013 2Document9 pagesCycloadditions Solutions 2013 2Ghadeer M HassanNo ratings yet
- Reactivity QuinolineDocument107 pagesReactivity QuinolineIan OttoNo ratings yet
- ThiopheneDocument398 pagesThiopheneAshwin MandaleNo ratings yet
- Cyclisations Solutions 2013Document7 pagesCyclisations Solutions 2013Ghadeer M HassanNo ratings yet
- Chapter 2Document39 pagesChapter 2Ghadeer M HassanNo ratings yet
- Heterocycles - PART 2 - Pall Knorr PyrroleDocument4 pagesHeterocycles - PART 2 - Pall Knorr PyrroleGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- Heterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsDocument4 pagesHeterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
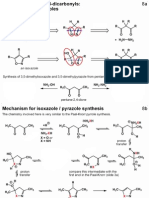
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocument4 pagesHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNo ratings yet
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocument4 pagesHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNo ratings yet
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- 2013 Hetero Model AnswerDocument2 pages2013 Hetero Model AnswerGhadeer M HassanNo ratings yet
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- Year 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestDocument4 pagesYear 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestGhadeer M HassanNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5782)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- KOHLER 17 GENERATOR 17servDocument100 pagesKOHLER 17 GENERATOR 17servRobert McKinley100% (2)
- KEYWORDS: Precipitation, Analytics, Volhard'S Method: AbstractDocument2 pagesKEYWORDS: Precipitation, Analytics, Volhard'S Method: AbstractThania GonzlezNo ratings yet
- Dryden Natural Springs Case AnalysisDocument3 pagesDryden Natural Springs Case Analysishanna100% (2)
- 16721-Fa SystemDocument17 pages16721-Fa SystemuddinnadeemNo ratings yet
- DPS200PB143Document1 pageDPS200PB143Techtrade ArgentinaNo ratings yet
- Inside The Worst-Hit County in The Worst-Hit State in The Worst-Hit Country - The New YorkerDocument17 pagesInside The Worst-Hit County in The Worst-Hit State in The Worst-Hit Country - The New YorkerMike DesaiNo ratings yet
- Cantonment Bye Law 2019 Dated 09 12 2019Document160 pagesCantonment Bye Law 2019 Dated 09 12 2019omerumeromer100% (2)
- Extra homework-Che-XDocument3 pagesExtra homework-Che-XBornil Bikash BhuyanNo ratings yet
- Vedic Mantras An Influential Factor For Spiritual HealthDocument7 pagesVedic Mantras An Influential Factor For Spiritual Healthbanny 123No ratings yet
- Monetary Choice Questionnaire, Extended Monetary Choice Questionnaire (MCQ-27, MCQ-36)Document1 pageMonetary Choice Questionnaire, Extended Monetary Choice Questionnaire (MCQ-27, MCQ-36)Elma SinghNo ratings yet
- Practice Problems - Groundwater Permeability and Seepage Part 2Document2 pagesPractice Problems - Groundwater Permeability and Seepage Part 2Kok Soon ChongNo ratings yet
- 3 Mission 35+ Vol. VIIDocument83 pages3 Mission 35+ Vol. VIINafis UnnisaNo ratings yet
- Lrfdcons 3 I3Document29 pagesLrfdcons 3 I3RAIMUNDO SUHERDINNo ratings yet
- Lactogrow VS Lactogen 2Document3 pagesLactogrow VS Lactogen 2Mardan Love SunnahNo ratings yet
- Aberrometry NotesDocument3 pagesAberrometry NotesVero VillamorNo ratings yet
- Biological Document-Papaya PDFDocument72 pagesBiological Document-Papaya PDFnuratika ikaNo ratings yet
- Procurement Management Plan TemplateDocument7 pagesProcurement Management Plan Templatekareem3456No ratings yet
- CC430 P2 air conditioning unit performance and specificationsDocument2 pagesCC430 P2 air conditioning unit performance and specificationsEdilson JunhoNo ratings yet
- Gsef 2021 Research Plan Project Summary InstructionsDocument1 pageGsef 2021 Research Plan Project Summary Instructionsapi-550508557No ratings yet
- Module 1 - Lecture 2Document32 pagesModule 1 - Lecture 2Bruce bannerNo ratings yet
- Role and Functions of Beneficial Microorganisms in Sustainable AquacultureDocument7 pagesRole and Functions of Beneficial Microorganisms in Sustainable Aquaculturepig_pink1989No ratings yet
- Oil Drilling Operation. Pre-DiggingDocument5 pagesOil Drilling Operation. Pre-DiggingКаролина ЛемешеваNo ratings yet
- CH 31 - Assessment and Management of Patients With Vascular Disorders and Problems of PeripheralDocument16 pagesCH 31 - Assessment and Management of Patients With Vascular Disorders and Problems of PeripheralPye Antwan DelvaNo ratings yet
- Bi Substrate ReactionsDocument14 pagesBi Substrate ReactionsIda Nancy KNo ratings yet
- Security in Project FinanceDocument25 pagesSecurity in Project FinanceIshaya AmazaNo ratings yet
- Written Works Mapeh 7&9 2ndDocument8 pagesWritten Works Mapeh 7&9 2ndNas LeeNo ratings yet
- ANSI AHRI Standard 850 I-P 2013Document16 pagesANSI AHRI Standard 850 I-P 2013Sergio Motta GarciaNo ratings yet
- Stages of Prophase 1: LeptoteneDocument3 pagesStages of Prophase 1: LeptotenepixiedustNo ratings yet
- Problems-Convection 1Document2 pagesProblems-Convection 1Ira Octavia100% (1)
- Plant PartsDocument49 pagesPlant Partsshahzad18400% (1)