Professional Documents
Culture Documents
CO1 Concrete Example ASR
Uploaded by
PetrolabCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CO1 Concrete Example ASR
Uploaded by
PetrolabCopyright:
Available Formats
A Bridge
A Client
Concrete Petrographic Report CO1
Example
Petrolab Limited
www.petrolab.co.uk
tel +44 (0)1209 219541 email petrolab@petrolab.co.uk
C Edwards Offices, Gweal Pawl, Redruth, Cornwall TR15 3AE
Registered in England & Wales Company No. 4777735
Petrographic Report - Concrete
A Client
Contents
1 Sample details..........................................................................................1
2 Petrographic description C1...................................................................2
2.1
2.2
2.3
2.4
2.5
Aggregates.............................................................................................................2
Binder....................................................................................................................3
Voids......................................................................................................................4
Fractures and degradation....................................................................................4
Composition - C1...................................................................................................5
3 Petrographic description C2...................................................................6
3.1
3.2
3.3
3.4
3.5
Aggregates.............................................................................................................6
Binder....................................................................................................................8
Voids....................................................................................................................10
Fractures and degradation..................................................................................11
Composition - C2.................................................................................................13
4 Summary.................................................................................................14
4.1 Concrete C1........................................................................................................14
4.2 Concrete C2........................................................................................................14
5 Images....................................................................................................15
5.1 Sample C1...........................................................................................................16
5.2 Sample C2...........................................................................................................19
A Bridge
CO1 Example
Issued by Petrolab Ltd
i
Petrographic Report - Concrete
A Client
Petrolab document control
Client
A Client
Report title
A Bridge
Analysis required
Detailed petrographic examination in accordance with ASTM C-856 on two
concrete cores.
Client reference
--
Client contact
A Client
Report ID (version)
CO1
Report date
Example
Prepared by
R Garside BA MSci
Checked by
J Strongman Msci ARSM
Limitations
This report relates only to those samples submitted and specimens examined and to any materials properly
represented by those samples and specimens. This report is issued to the Client named above for the benefit of
the Client for the purposes for which it was prepared. It does not confer or purport to confer on any third party any
benefit or right pursuant to the Contracts (Rights of Third Parties) Act 1999.
A Bridge
CO1 Example
Issued by Petrolab Ltd
ii
Petrographic Report - Concrete
A Client
Petrographic methods of investigation for hardened concrete
Preliminary examination
The submitted sample / specimens are examined as received, and after careful washing to remove
loose debris, using a Nikon SMZ-U stereomicroscope. The microscope has a continuous zoom range
of 7.5x to 75x and it is equipped with a 150W continuous ring, fibre optic illuminator. It is useful for the
preliminary examination of materials and it is possible to discriminate and sometimes identify features
as small as 100 m in size. The microscope has a trinocular head and can be used for low power
photomicrography. The main purpose of the preliminary examination is to identify the degree of
consistency or variability in the submitted sample / specimens and any superficial evidence of
deterioration. This facilitates the selection of the most appropriate specimen(s) for a full petrographic
examination when multiple specimens of the same concrete type are submitted.
Sample preparation
Following preliminary examination, the selected specimen is cut in half parallel or perpendicular with its
axis (to suit the objectives of the investigation). One half of a cut specimen may be carefully ground,
finishing with #600 grit carborundum powder. At least one standard petrographic thin section is
prepared from each selected specimen. Additional thin sections may be prepared from a large
specimen in order to investigate variation in the concrete or to suit the objectives of the investigation.
The cut specimen, used for section preparation, is typically vacuum impregnated with a low viscosity
epoxy resin containing a yellow fluorescent dye. A high resolution, low magnification digital image of
each thin section is prepared using a film scanner. This type of image is useful for illustrating the
mesostructure of the concrete.
Microscopic examination
High-power microscopy is undertaken using a Nikon research polarising microscope. The thin section(s)
may be examined in both plain and cross-polarised light, and reflected ultraviolet light creating
secondary fluorescence. Photomicrographs are taken using a Nikon high resolution digital camera fitted
to the trinocular head of the microscope.
Quantitative investigations
Modal analysis to determine the concrete composition is performed using a Pelcon 64 channel
electromechanical point counter using stepping and traverse intervals of 0.5 mm. One thousand points
are counted from each thin section (provided the area of the sample represented on the thin-section is
sufficient). Water-cement ratios for uncarbonated concrete can be estimated by the petrographic
comparison of the fluorescent intensity of the paste with that of standard mortars viewed under carefully
controlled reflected ultraviolet illumination. An estimated mix design may be calculated for standard
Portland-type cement without cement substitutes.
Other testing
In some circumstances, additional (non-petrographic) testing using an appropriate chemical or physical
testing procedure may be undertaken by an approved laboratory. When undertaken, the test certificate
and the results obtained are provided in an appendix to the main body of the report.
A Bridge
CO1 Example
Issued by Petrolab Ltd
iii
Petrographic Report - Concrete
A Client
Sample details
Two samples of concrete were supplied by A Client on xx/xx/xxxx for petrographic
examination. The samples were reported to be from a concrete bridge structure.
Sample details, as received, are shown in the table below.
Table 1 Samples received
Report no.
Sample reference
Type
C1
Core location
Concrete core, 100 mm
C2
Core location
Concrete core, 100 mm
The investigation requested was a detailed petrographic description and quantitative
modal analysis, to assess concrete quality and any evidence of degradation, in
particular any evidence or potential for Alkali-Silica Reaction (ASR). A thin section was
made parallel to the core axis in the area of the core which showed the greatest
evidence of degradation, or, when there was no immediately evident damage, the base
of the core as this would be most likely to show the presence of ASR.
Two types of concrete were identified. Petrographic observations are recorded in the
tables below with additional explanatory text beneath. A summary of the concrete
type(s) is provided followed by annotated images of the samples.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 1 of 21
Petrographic Report - Concrete
A Client
Petrographic description C1
2.1
Aggregates
Table 2 Aggregate details
crushed aggregate and natural sand
Type
Coarse aggregate crushed limestone
Abundance
Major components
bioclastic limestone
Minor components
calcareous shell fragments
Size range
3 mm
Grading
good
to
20 mm
47.4%
Average
10 mm
Rounding
angular sub-angular
angular morphologies predominate
Shape
mainly equant
Fine aggregate
beach sand
Major components
vein quartz, calcareous shell fragments
Minor components
limestone, ferruginous sandstone, stable silicate minerals (plagioclase feldspar, Kfeldspar, pyroxene), altered basalt, iron oxides, calcite, chert, zircon, glauconite,
volcanic glass
Size range
50 m
Grading
good
to
Sphericity
low
Abundance
1 mm
22.1%
Average
300 m
Rounding
sub-angular - rounded
sub-rounded morphologies predominate
Shape
mainly equant
Percentage passing 600 m
Sample
C1
Sphericity
moderate
80 (visual estimate)
Aggregate microfractures
Secondary alkali-silica gel
none seen
none seen
The coarse aggregate is a crushed limestone and the fine aggregate is a beach sand.
Both aggregates are generally considered stable. The fine aggregate contains a minor
amount of chert and volcanic glass (2.7 % of the aggregate total, modal analysis) which
are considered potential alkali-silica reactive components 1. However the aggregate in
this sample shows no evidence of alkali-silica gel development or associated radial
microfracturing.
There is no evidence of any other aggregate instability or reaction between aggregate
and the binder.
1 The Diagnosis of Alkali-Silica Reaction, Report of a working party. Appendix D. British Cement
Association, 1992. Where rocks or mineral types are noted as 'reactive', it does not necessarily imply
that damage has been caused by ASR when these have been used.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 2 of 21
Petrographic Report - Concrete
2.2
A Client
Binder
The binder is Portland-type cement, probably OPC. The sample appears to be
completely uncarbonated except for trace patchy carbonation in the surface 10 mm of
the concrete.
The optical properties of the uncarbonated paste are summarised in the table below.
Table 3 Uncarbonated binder
Cement type
Portland-type Cement (probably OPC)
Replacement
local ettringite replacement
Modal volume
29.8%
Optical properties of least altered paste
Colour
dark brown to pale fawn
Isotropy
weakly anisotropic
Texture
irregularly mottled
Disseminated calcite abundant
Dye absorption
variable, moderate to high
Fluorescence
variable, moderate to high
abundant
Distribution
irregular
Shape
granular and branching
anhedral grains
Aggregate margins common
Distribution
irregular
Maximum size (m) 80
Shape
monolayers and outgrowths
Portlandite
In paste
Maximum size (m) 60
Replacement
Replacement
calcite (common)
calcite, ettringite (rare)
Cement clinker grains
Alite
common
Maximum size (m)
100
Max. birefringence
not determined
Colour
pale yellow
Hydration
CSH-replaced
Reaction rims
none seen
Belite
rare
Maximum size (m)
30
Max. birefringence
not determined
Colour
muddy brown
Hydration
CSH-replaced
Reaction rims
diffuse opaque rims
Ferrite
present
Maximum size (m)
50
Alteration
strongly oxidised
Colour
dark red-brown - opaque
Maximum size (m)
200
Clinker grain clusters
Alite
common
Matrix
dark brown glassy phase, granular iron oxide
Hydration
completely hydrated
Reaction rims
none seen
Belite
rare
Maximum size (m)
150
Matrix
sparse, medium brown glassy phase
Hydration
strongly hydrated
Alite + belite
none seen
Reaction rims
diffuse opaque rims
The mineralogy and texture of the uncarbonated paste are characteristic of Portland
Cement concrete made at a moderate water/cement ratio ( 0.45). The uncarbonated
binder shows evidence of minor local ettringite replacement.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 3 of 21
Petrographic Report - Concrete
2.3
A Client
Voids
The main features of the air void system are summarised below.
Table 4 Voids
Void system
Modal volume
0.7%
Size range
100 m to
Interconnectivity
generally isolated
5 mm
Shape
spherical irregular
Average size
500 m
Secondary minerals
ettringite
present
Alteration at void walls
local ettringite replacement
There are traces of ettringite in voids and the surrounding binder, particularly in areas of
slightly higher porosity, however there is no evidence of significant sulphate attack.
2.4
Fractures and degradation
Table 5 Fractures
Anastomosing microcracks
Abundance
none seen
Peripheral fissures
Abundance
rare
Distribution
Maximum aperture
Secondary minerals
Mineral 1
Mineral 2
Mineral 3
at margins of aggregate fragments, no specific lithologies
Average aperture 35 m
60 m
Alteration at crack walls
local ettringite replacement
granular calcite
portlandite
ettringite
Abundance
Abundance
Abundance
rare
present
present
Fractures related to degradation
Abundance
none seen
Peripheral fissures may form as a result of aggregate shrinkage and as a result of
dissolution or ettringite replacement of portlandite outgrowths and monolayers.
The samples show no evidence of microfracturing related to sulphate replacement of
the binder or other aggregate-related degradation.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 4 of 21
Petrographic Report - Concrete
2.5
A Client
Composition - C1
The volumetric composition of the concrete was determined by modal analysis. One
thousand points were counted from the thin section.
Sample
C1
Coarse aggregate
47.4%
Fine aggregate
22.1%
Binder
29.8%
Voids
0.7%
Water-cement ratio was estimated by comparing the fluorescent intensity of the paste
under high intensity reflected ultraviolet illumination with that of standard mortars. A
range of values between 0.4 and 0.5 were obtained. A preferred water-cement ratio of
0.45 has been used to estimate the mix design.
Table 6 Estimated (theoretical) mix design
Volume
%
Density
(kgm3)
Coarse aggregate
47.4
2650
Coarse aggregate
1256 kgm3
Sand aggregate
22.1
2650
Sand aggregate
586
kgm3
Binder
29.8
3120
Cement
387
kgm3
Voids
0.7
Water
174
kgm3
Total
100.0
Density
2403 kgm3
Slump without plasticiser
30-60 mm
28-day strength
51.7
Component
Fluorescent intensity
Preferred water/cement ratio
Maximum aggregate size, mm
0.4-0.5
0.45
20
crushed
Estimated mix design
w/c
0.45
Calculated cement content 16.1
MPa
%
The estimate assumes that no air entrainment agent or plasticiser is present.
NOTE: It must be emphasised that the estimation of concrete mix design by petrographic methods is
subject to many potential errors. It should not be used as a substitute for appropriate and approved
methods of chemical analysis and physical testing.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 5 of 21
Petrographic Report - Concrete
A Client
Petrographic description C2
3.1
Aggregates
Table 7 Aggregate details
crushed aggregate and natural sand
Type
Coarse aggregate crushed limestone
Abundance
Major components
bioclastic limestone, carbonaceous limestone (silicified limestone?)
Minor components
greywacke, sandstone
Size range
2 mm
Grading
good
to
20 mm
53.0%
Average
10 mm
Rounding
angular sub-angular
angular morphologies predominate
Shape
mainly equant
Fine aggregate
beach sand
Major components
vein quartz, calcareous shell fragments
Minor components
limestone, ferruginous sandstone, stable silicate minerals (plagioclase feldspar, Kfeldspar, pyroxene), altered basalt, iron oxides, calcite, chert, zircon, glauconite,
volcanic glass
Size range
50 m
Grading
good
to
Sphericity
low
Abundance
1 mm
18.7%
Average
300 m
Rounding
sub-angular - rounded
sub-rounded morphologies predominate
Shape
mainly equant
Percentage passing 600 m
Sample
C2
Sphericity
moderate
80 (visual estimate)
Aggregate microfractures
Secondary alkali-silica gel
common - generally gel-filled, iron
oxide filled near base of sample
common, sometimes carbonated
The coarse aggregate is a crushed limestone and the fine aggregate is a beach sand.
The fine aggregate contains a minor amount of chert and volcanic glass (2.5 % of the
aggregate total, modal analysis) and the coarse aggregate contains a minor amount of
greywacke (< 5 % of the aggregate total, visual estimate). These are considered
potential alkali-silica reactive components 1 but show no evidence of initiating ASR in the
thin section.
In this sample < 20 % coarse aggregate (visual estimate) shows evidence of internal
fracturing and the development of pervasive cracking and alkali-silica gel formation. The
binder around these aggregate fragments also shows significant gel replacement, in
patches < 2 mm.
In this sample the coarse aggregate most commonly associated with fracturing and
surrounding gel-filled binder is the carbonaceous limestone. This aggregate type is not
typically considered a high risk ASR aggregate, as ASR is usually attributed to the
presence of microcrystalline or highly strained quartz. Although the limestone does not
appear to be obviously silicified in thin section, it does contain small strained and finely
1 The Diagnosis of Alkali-Silica Reaction, Report of a working party. Appendix D. British Cement
Association, 1992. Where rocks or mineral types are noted as 'reactive', it does not necessarily imply
that damage has been caused by ASR when these have been used.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 6 of 21
Petrographic Report - Concrete
A Client
crystalline quartz grains and the abundant opaque carbonaceous material may also
contain microcrystalline silica which is not distinguishable under the microscope. The
aggregate fragments are harder than typical limestone which would also suggest that
they have been silicified.
Silicified limestone is not widely considered a high risk aggregate and only a small
number of cases of reaction with this aggregate have been reported in the UK. It is
possible that some unusual environmental conditions, such as high alkali cement, may
have caused the aggregate to react.
There is no evidence of any other aggregate instability or reaction between aggregate
and the binder.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 7 of 21
Petrographic Report - Concrete
3.2
A Client
Binder
The binder is Portland-type cement, probably OPC. The core is completely
uncarbonated except for a strong carbonation layer around some fractures and patchy
carbonation of alkali-silica gel.
The optical properties of the uncarbonated paste are summarised in the table below.
Table 8 Uncarbonated binder
Cement type
Portland-type Cement (probably OPC)
Replacement
local ettringite, alkali-silica gel, iron oxide
Modal volume
27.7%
Optical properties of least altered paste
Colour
pale fawn to dark brown
Isotropy
weakly anisotropic
Texture
irregularly mottled
Disseminated calcite abundant
Dye absorption
variable, moderate to high
Fluorescence
variable, moderate to high
abundant
Distribution
irregular
Shape
granular and branching
anhedral grains
Aggregate margins common
Distribution
irregular
Maximum size (m) 110
Shape
tabular crystals and
outgrowths
Portlandite
In paste
Maximum size (m) 50
Replacement
Replacement
calcite (common)
calcite, ettringite (rare)
Cement clinker grains
Alite
common
Maximum size (m)
100
Max. birefringence
not determined
Colour
pale yellow
Hydration
CSH-replaced
Reaction rims
none seen
Belite
rare
Maximum size (m)
40
Max. birefringence
not determined
Colour
muddy brown
Hydration
CSH-replaced
Reaction rims
diffuse opaque rims
Ferrite
present
Maximum size (m)
75
Alteration
strongly oxidised
Colour
dark red-brown - opaque
Maximum size (m)
100
Clinker grain clusters
Alite
common
Matrix
dark brown glassy phase, granular iron oxide
Hydration
completely hydrated
Reaction rims
none seen
Belite
rare
Maximum size (m)
140
Matrix
sparse, medium brown glassy phase
Hydration
strongly hydrated
Reaction rims
diffuse opaque rims
Alite + belite
rare
Maximum size (m)
165
Matrix
medium brown glassy phase, granular iron oxide
Hydration
strongly hydrated
Reaction rims
diffuse opaque rims
The mineralogy and texture of the uncarbonated paste are characteristic of Portland
Cement concrete made at a moderate water-cement ratio ( 0.5). The uncarbonated
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 8 of 21
Petrographic Report - Concrete
A Client
binder shows evidence of minor local ettringite replacement. It also shows evidence of
patchy alkali-silica gel replacement around aggregate fragments and iron oxide
replacement at the base of the core, presumably in proximity to corroded reinforcement.
The main features of the carbonated paste are summarised below.
Table 9 Carbonated binder
Transition zones
Carbonation fronts
sharp, irregular
Width of transition zone
100 m 300 m
Replacement sequence
portlandite
paste
cement clinker grains
Fine-grained paste
Abundance
present
Size range
< 1 m
Relict clinker grains
present
Residual gel
present
Granular iron oxide present
Maximum size
50 m
Maximum size
50 m
Microporosity
low
Pore size
5 m
Secondary minerals
ettringite, alkali-silica gel
Distribution
patches next to corroded
reinforcement
Texture
microgranular
to
5 m
Distribution
around microcracks and areas
of carbonated gel
Texture
uniform, compact
To
25 m
Coarse-grained paste
Abundance
present
Size range
5 m
Relict clinker grains
present (alite)
Residual gel
present
Granular iron oxide present
Maximum size
100 m
Maximum size
100 m
Microporosity
high locally
Pore size
5 m
Secondary minerals
ettringite, iron oxides
Snowflake calcite
none seen
to
25 m
to
50 m
The carbonated paste around gel-associated fractures is mainly fine-grained and
strongly recrystallised. The texture is typical of natural atmospheric carbonation along
high porosity zones. Carbonated paste around the iron oxide filled binder is coarse
grained, suggesting that fractures penetrating down to the reinforcement have acted as
pathways for leaching in addition to facilitating corrosion of the steel.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 9 of 21
Petrographic Report - Concrete
3.3
A Client
Voids
The main features of the air void system are summarised below.
Table 10 Voids
Void system
Modal volume
0.6%
Size range
50 m
Interconnectivity
locally highly connected
to
5 mm
Shape
spherical irregular
Average size
500 m
Secondary minerals
Voids in uncarbonated paste
Voids in carbonated paste
ettringite
rare
granular calcite
rare
portlandite
rare
ettringite
rare
alkali-silica gel
rare
alkali-silica gel
rare
iron oxides
rare
Alteration at void walls
local ettringite or alkali-silica gel replacement
There are traces of ettringite in voids and the surrounding binder, particularly in areas of
slightly higher porosity, however there is no evidence of significant sulphate attack.
Voids around the edge of gel filled binder or fractures show evidence of gel linings and
voids next to the iron oxide filled binder also contain iron oxides.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 10 of 21
Petrographic Report - Concrete
3.4
A Client
Fractures and degradation
Table 11 Fractures
Anastomosing microcracks
Abundance
none seen
Peripheral fissures
Abundance
rare
Distribution
around coarse aggregate fragments, no specific lithologies
Maximum aperture
30 m
Average aperture
15 m
Mineral 1
ettringite
Abundance
present
Mineral 2
portlandite
Abundance
present
Mineral 3
alkali-silica gel
Abundance
rare
Mineral 4
iron oxides
Abundance
rare
Mineral 5
granular calcite
Abundance
rare
Secondary minerals
Alteration at crack walls ettringite, iron oxide binder replacement
Fractures related to degradation
Abundance
common
Type 1 throughout core sample, no specific orientation
Distribution
Type 2 at base of sample, generally sub-parallel to surface
Maximum aperture
1 1.2 mm; 2 100 m Average aperture 1 200 m; 2 - 25 m
usually closely matching in binder and aggregate, occasionally non-matching in
Crack walls
carbonaceous limestone aggregate
cracks commonly run through and possibly propagate in carbonaceous
Cracks in aggregate
limestone aggregate fragments
Secondary minerals
Mineral 1
ettringite
Abundance
common
Mineral 2
alkali-silica gel
Abundance
common
Mineral 3
iron oxides
Abundance
common
granular calcite
Abundance
present
Mineral 4
Alteration at crack walls ettringite, alkali-silica gel, iron oxide binder replacement
Peripheral fissures may form as a result of aggregate shrinkage and as a result of
dissolution or ettringite replacement of portlandite outgrowths and monolayers.
Fractures related to development of alkali-silica gels mostly propagate into the thin
section from outside the area, though there are a few microfractures which appear to
originate in carbonaceous limestone fragments. These discontinuous fractures
permeate into the surrounding paste approximately perpendicular to the aggregate
surface and can be traced for up to at least 20 mm. Fractures in the binder generally
have matching walls with discontinuous linings of alkali-silica gel and/or lining of fibrous
ettringite. Maximum fracture apertures are < 1.2 mm but are typically 200 m in width.
The gel in the majority of fractures is uncarbonated, except for the wider fractures and
areas with gel replacing the binder. It appears to be at least partly contemporaneous
with the ettringite, since it both overgrows ettringite in fractures and has ettringite
forming on its surface.
The most likely cause of the ASR development is due to the concrete being
continuously wet which allows the alkalinity of the pore fluid to increase to a point where
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 11 of 21
Petrographic Report - Concrete
A Client
any microcrystalline and/or highly strained quartz reacts. The presence of sulphate
replacement in the uncarbonated binder is further evidence that the concrete has been
wet for prolonged periods. The presence of carbonated gel and evidence that ASR
fracturing has caused reinforcement corrosion suggests that some of the gel is relatively
old. However, it is not possible to determine whether alkali-silica reaction in the concrete
has ceased or is ongoing.
Observations of the core sample show evidence of corrosion and associated expansion
of steel reinforcement at the base. This has produced multiple radiating surface-parallel
microfractures which are iron oxide-filled and there is local iron oxide impregnation of
the binder (< 3 mm from sample base). The cause of corrosion is due to air ingress and
subsequent carbonation. The process is a classic feedback system: as the steel
becomes more corroded it expands further creating more fractures which penetrate to
the surface allowing further air ingress and corrosion.
The core sample provided is almost completely uncarbonated and the concrete is
extremely dense. It is considered unlikely that surface carbonation has penetrated to the
depth of the reinforcement and, therefore there must have been an initial fracture
mechanism to create a pathway to the reinforcement. From the evidence of carbonated
alkali-silica gels in fractures it seems likely that the initial fracturing was caused by ASR.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 12 of 21
Petrographic Report - Concrete
3.5
A Client
Composition - C2
The volumetric composition of the concrete was determined by modal analysis. One
thousand points were counted from the thin section. The concrete appears to be slightly
unevenly mixed as there are patches of high cement content with abundant unhydrated
clinker and other areas with a higher water content.
Sample
C2
Coarse aggregate
53.0%
Fine aggregate
18.7%
Binder
27.7%
Voids
0.6%
Water-cement ratio was estimated by comparing the fluorescent intensity of the paste
under high intensity reflected ultraviolet illumination with that of standard mortars. A
range of values between 0.45 and 0.55 were obtained. A preferred water-cement ratio of
0.5 has been used to estimate the mix design.
Table 12 Estimated (theoretical) mix design
Volume
%
Density
(kgm3)
Coarse aggregate
53.0
2650
Coarse aggregate
1405 kgm3
Fine aggregate
18.7
2650
Sand aggregate
496
kgm3
Binder
27.7
3120
Cement
338
kgm3
Voids
0.6
Water
169
kgm3
Total
100.0
Density
2406 kgm3
Slump without plasticiser
30-60 mm
28-day strength
45.5
Component
Fluorescent intensity
0.45-0.55
Preferred water/cement ratio
0.5
Maximum aggregate size, mm
20
crushed
Estimated mix design
w/c
0.5
Calculated cement content 14.0
MPa
%
The estimate assumes that no air entrainment agent or plasticiser is present
NOTE: It must be emphasised that the estimation of concrete mix design by petrographic methods is
subject to many potential errors. It should not be used as a substitute for appropriate and approved
methods of chemical analysis and physical testing.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 13 of 21
Petrographic Report - Concrete
Summary
4.1
Concrete C1
A Client
1. One core sample of concrete was investigated to assess concrete quality and any
evidence of degradation. A thin section was prepared parallel with the long axis of the
core from an area near the base of the sample provided.
2. The cement appears to be Ordinary Portland Cement; there is no petrographic evidence
of any admixtures or air entrainment.
3. The estimated mix design appears to be within normal limits.
4. The coarse aggregate is composed of crushed limestone.
5. The fine aggregate is a beach sand.
6. Chert and volcanic glass make up circa 2.7 % of the total aggregate (modal analysis).
Chert and volcanic glass are considered potentially alkalisilica reactive components.
However, chert and glass in this sample show no evidence of alkali-silica reaction and/or
fracturing.
7. There is no evidence of any other aggregate instability or aggregate binder reaction in
the sample.
8. The sample appears to be completely uncarbonated except for trace patchy carbonation
in the surface 10 mm of the concrete.
9. Carbonation of the concrete is not a problem in itself as it does not significantly alter the
strength of the concrete. However, it is a potentially serious problem if the carbonation
penetrates to the depth of steel reinforcement, where it may facilitate corrosion and
associated expansion of the steel.
10. No reinforcement was observed in this sample.
11. There are traces of secondary sulphates in some voids and peripheral microfractures
and the surrounding binder, particularly in areas of slightly higher porosity, however there
is no evidence of significant sulphate attack.
12. The sample shows no evidence of microfracturing related to sulphate replacement of the
binder or other aggregate-related degradation.
13. The concrete in this sample shows no evidence of macroscopic fracturing relating to
degradation and appears to be currently sound.
4.2
Concrete C2
1. One core sample of concrete was investigated to assess concrete quality and any
evidence of degradation. A thin section was prepared parallel with the long axis of the
core from an area which appeared to show the most evidence of degradation.
2. The cement appears to be Ordinary Portland Cement; there is no petrographic evidence
of any admixtures or air entrainment.
3. The estimated mix design appears to be within normal limits.
4. The coarse aggregate is composed of crushed limestone.
5. The fine aggregate is a beach sand.
6. The fine aggregate contains a minor amount of chert and volcanic glass (circa 2.5 % of
the aggregate total, modal analysis) and the coarse aggregate contains a minor amount
of greywacke (< 5 % of the aggregate total, visual estimate). Chert, volcanic glass and
greywacke are considered potentially alkalisilica reactive components. However, in this
sample these components show no evidence of alkali-silica reaction and/or fracturing.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 14 of 21
Petrographic Report - Concrete
A Client
7. Carbonaceous limestone aggregate fragments shows evidence of internal fracturing and
the development of radial cracking and alkali silica gels. Although limestone aggregates
are generally considered to be of low reactivity, there have been some cases where
silicification of limestones has been identified as the source of deleterious ASR. Silicified
limestone is not widely considered a high risk aggregate and only a small number of
cases of reaction with this aggregate have been reported in the UK. It is possible that
some unusual environmental conditions, such as high alkali cement, may have caused
the aggregate to react.
8. Fractures related to development of alkali-silica gels in the sample show patchy
distribution through the core sample. The microfractures typically contain discontinuous
linings and fills of alkali-silica gel and fibrous ettringite, with gel usually overgrowing the
sulphates (though in some places sulphate also overgrows gel). The binder around some
carbonaceous aggregate samples also shows significant gel replacement, in patches
< 2 mm.
9. The most likely cause of the ASR development is due to the concrete being continuously
wet which allows the alkalinity of the pore fluid to increase to a point where any
microcrystalline and/or highly strained quartz reacts. The presence of sulphate
replacement in the uncarbonated binder is further evidence that the concrete has been
wet for prolonged periods. The presence of carbonated gel and evidence that ASR
fracturing has caused reinforcement corrosion suggests that some of the gel is relatively
old. However, it is not possible to determine whether alkali-silica reaction in the concrete
has ceased or is ongoing.
10. There are traces of secondary sulphates, alkali-silica gel linings and iron oxides in some
voids, peripheral microfractures and the surrounding binder, particularly in areas of
slightly higher porosity, however there is no evidence of significant sulphate attack.
11. The core is completely uncarbonated except for a strong carbonation layer around some
fractures and patchy carbonation of alkali-silica gel.
12. Carbonation of the concrete is not a problem in itself as it does not significantly alter the
strength of the concrete. However, it is a potentially serious problem if the carbonation
penetrates to the depth of steel reinforcement, where it may facilitate corrosion and
associated expansion of the steel.
13. Three 2 mm reinforcement bars were observed in the core sample at 35-42 mm depth.
These pieces of reinforcement show no evidence of corrosion or microfracturing. The
core also contains evidence of steel reinforcement at a depth of 60 mm from the surface.
The reinforcement is extremely corroded and shows multiple radiating sub horizontal
microfractures, the fractures are iron oxide filled and there is locally strong iron oxide
impregnation of the binder.
14. The cause of corrosion is due to air ingress and carbonation. The process has a positive
feedback because as the steel becomes more corroded it expands further creating more
fractures, thus allowing further air ingress and corrosion of the steel.
15. Due to the evidence of degradation from both ASR and reinforcement
sample provided, the concrete and the associated structure requires
damage and ongoing close monitoring. It is recommended that the
concrete in proximity to any reinforcement be ascertained because of
corrosion observed.
corrosion in the
assessment for
condition of the
the evidence of
Images
Colour images (scanned images and annotated photomicrographs of each sample)
begin over-page.
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 15 of 21
Petrographic Report - Concrete
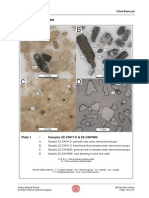
5.1
A Client
Sample C1
A
Sample C1
Photograph of sample as received
(scale in cm).
Image A
Fuji S3 Pro digital camera
Daylight balanced oblique light
Thin section(s)
B
Sample C1
Low magnification view of sample thin
section. (Orientation arrow points
towards drilling surface.)
Image B
Epson scanner
White cold cathode light
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 16 of 21
Petrographic Report - Concrete
A Client
Photomicrographs
C
shell
Sample C1
General view of sample showing all
main phases.
limestone
void
Image C
Nikon Microphot-FXA petrological
microscope
Plane polarised transmitted light
x40
quartz
shell
500 m
D
shell
Sample C1
View of image C in cross polarised
light.
limestone
void
Image D
Nikon Microphot-FXA petrological
microscope
Cross polarised transmitted light
x40
quartz
shell
500 m
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 17 of 21
Petrographic Report - Concrete
A Client
E
Sample C1
Image showing minor ettringite in
voids.
void
ettringite
Image E
Nikon Microphot-FXA petrological
microscope
Plane polarised transmitted light
x200
ettringite
100 m
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 18 of 21
Petrographic Report - Concrete
5.2
A Client
Sample C2
A
Sample C2
Photograph of sample as received
(scale in cm).
Image A
Fuji S3 Pro digital camera
Daylight balanced oblique light
Thin section(s)
B
Sample C2
Low magnification view of sample thin
section. (Orientation arrow points
towards drilling surface.)
Image B
Epson scanner
White cold cathode light
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 19 of 21
Petrographic Report - Concrete
A Client
Photomicrographs
C
Sample C2
Image showing corrosion induced
microfracturing and iron oxide
impregnation of binder at the base of
the core.
iron oxides
limestone
microfracture
Image C
Nikon Microphot-FXA petrological
microscope
Plane polarised transmitted light
x40
quartz
iron oxides
500 m
Sample C2
Image showing an area of alkali-silica
gel completely replacing the binder
around carbonaceous limestone
aggregate.
Image D
Nikon Microphot-FXA petrological
microscope
Plane polarised transmitted light
x100
gel
quartz
carbonaceous
limestone
250 m
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 20 of 21
Petrographic Report - Concrete
A Client
E
Sample C2
Image showing alkali silica gel and
ettringite within microfractures. An
area of gel replacing the binder is also
visible.
gel
Image E
Nikon Microphot-FXA petrological
microscope
Plane polarised transmitted light
x200
gel
microfracture
gel
ettringite
100 m
A Bridge
CO1 Example
Issued by Petrolab Ltd
Page: 21 of 21
You might also like
- Price List 2019 SectionsDocument1 pagePrice List 2019 Sections4015johnfNo ratings yet
- Mineral Liberation StudyDocument37 pagesMineral Liberation StudyPetrolabNo ratings yet
- Bulk Mineral Analysis of Heavy Mineral SandsDocument27 pagesBulk Mineral Analysis of Heavy Mineral Sands4015johnfNo ratings yet
- OP3 Slate ExampleDocument10 pagesOP3 Slate ExamplePetrolabNo ratings yet
- A Compendium of Concrete Aggregates Used in SW EnglandDocument136 pagesA Compendium of Concrete Aggregates Used in SW EnglandPetrolabNo ratings yet
- UC3 Settings Controls 20-04-2015 DraftDocument4 pagesUC3 Settings Controls 20-04-2015 DraftPetrolabNo ratings yet
- Op2 Simplified Aggregate Example SandDocument1 pageOp2 Simplified Aggregate Example SandPetrolabNo ratings yet
- MP1 Optical Example PB SlagDocument8 pagesMP1 Optical Example PB SlagPetrolabNo ratings yet
- OP1 Rock Example DoleriteDocument14 pagesOP1 Rock Example DoleritePetrolab100% (1)
- Canadian Tungsten PlatesDocument1 pageCanadian Tungsten PlatesPetrolabNo ratings yet
- Heavy Mineral Sands PlatesDocument4 pagesHeavy Mineral Sands PlatesPetrolabNo ratings yet
- Canadian Tungsten PagesDocument2 pagesCanadian Tungsten PagesPetrolabNo ratings yet
- Heavy Mineral Sands PagesDocument11 pagesHeavy Mineral Sands PagesPetrolabNo ratings yet
- Materials Preparation Order FormDocument2 pagesMaterials Preparation Order FormPetrolabNo ratings yet
- Silica Sand Contamination PlatesDocument6 pagesSilica Sand Contamination PlatesPetrolabNo ratings yet
- Jurassic Sandstone PagesDocument1 pageJurassic Sandstone PagesPetrolabNo ratings yet
- Turkish Carbonate PlatesDocument1 pageTurkish Carbonate PlatesPetrolabNo ratings yet
- Columbian Gold PlatesDocument1 pageColumbian Gold PlatesPetrolabNo ratings yet
- Clay Matrix: Plate 1: (Sample Ref Removed)Document1 pageClay Matrix: Plate 1: (Sample Ref Removed)PetrolabNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Assessing Student Learning OutcomesDocument20 pagesAssessing Student Learning Outcomesapi-619738021No ratings yet
- Chapter 1 - The Empirical Beginnings and Basic Contents of Educational PsychologyDocument9 pagesChapter 1 - The Empirical Beginnings and Basic Contents of Educational PsychologyJoshua Almuete71% (7)
- Bibliography PresocraticsDocument10 pagesBibliography Presocraticsalraun66No ratings yet
- PMMC ExperimentDocument2 pagesPMMC ExperimentShyam ShankarNo ratings yet
- Optitex Com Products 2d and 3d Cad SoftwareDocument12 pagesOptitex Com Products 2d and 3d Cad SoftwareFaathir Reza AvicenaNo ratings yet
- Absolute Value - WikipediaDocument10 pagesAbsolute Value - WikipediaVenu GopalNo ratings yet
- EA Flora 1Document3 pagesEA Flora 1A. MagnoNo ratings yet
- Haier's Performance Management in Other CulturesDocument8 pagesHaier's Performance Management in Other CulturesSubhransu SahooNo ratings yet
- Open MPDocument30 pagesOpen MPmacngocthanNo ratings yet
- De HSG Lay Cau - Ban WordDocument124 pagesDe HSG Lay Cau - Ban WordNguyễn Hải YếnNo ratings yet
- OB HandoutsDocument16 pagesOB HandoutsericNo ratings yet
- Liugong 938 Wheel Loader Parts ManualDocument20 pagesLiugong 938 Wheel Loader Parts Manualjonathan100% (49)
- Why Leaders Should Look in the “MirrorDocument4 pagesWhy Leaders Should Look in the “MirrorCaryl Baylon EstreraNo ratings yet
- My ResumeDocument4 pagesMy Resumeapi-216740002No ratings yet
- British and American Culture Marking RubricDocument5 pagesBritish and American Culture Marking RubricAn Ho LongNo ratings yet
- Component 2 Learner Statement Y2Document6 pagesComponent 2 Learner Statement Y2api-426152133No ratings yet
- Makerwys - Exe Version 4.891: by Pete Dowson © 2019 InstructionsDocument11 pagesMakerwys - Exe Version 4.891: by Pete Dowson © 2019 InstructionsRafrol RamonNo ratings yet
- Biosynthesis of FlavoursDocument9 pagesBiosynthesis of FlavoursDatta JoshiNo ratings yet
- Lorain Schools CEO Finalist Lloyd MartinDocument14 pagesLorain Schools CEO Finalist Lloyd MartinThe Morning JournalNo ratings yet
- Catalogue MinicenterDocument36 pagesCatalogue Minicentermohamed mahdiNo ratings yet
- Linear Programming Models: Graphical and Computer MethodsDocument91 pagesLinear Programming Models: Graphical and Computer MethodsFaith Reyna TanNo ratings yet
- Event ReportDocument2 pagesEvent Reportakshitdaharwal997No ratings yet
- English ProjectDocument10 pagesEnglish ProjectHarshman Singh HarshmanNo ratings yet
- Classification of MatterDocument2 pagesClassification of Matterapi-280247238No ratings yet
- Assignment No.7Document2 pagesAssignment No.7queen estevesNo ratings yet
- ccpc15 Supportive and Preventive WorkbookDocument30 pagesccpc15 Supportive and Preventive WorkbookJeremy HamptonNo ratings yet
- Product Differentiation and Market Segmentation As Alternative Marketing StrategiesDocument7 pagesProduct Differentiation and Market Segmentation As Alternative Marketing StrategiesCaertiMNo ratings yet
- Applied Physics Mini Launcher Lab ReportDocument12 pagesApplied Physics Mini Launcher Lab ReportTalharashid RamzanNo ratings yet
- New Microwave Lab ManualDocument35 pagesNew Microwave Lab ManualRadhikaNo ratings yet
- Rock ClimbingDocument11 pagesRock ClimbingDaria TurdalievaNo ratings yet