Professional Documents
Culture Documents
MRI Correlate
Uploaded by
fadhlialbaniCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MRI Correlate
Uploaded by
fadhlialbaniCopyright:
Available Formats
ORIGINAL CONTRIBUTION
Clinical and MRI Correlates of Cerebral Palsy
The European Cerebral Palsy Study
Martin Bax, DM, FRCPCH
Clare Tydeman, BA(Hons)
Olof Flodmark, MD, PhD
HIS ARTICLE REPORTS ON THE
European Cerebral Palsy Study,
carried out in 8 centers in Europe, including North West
London and North East London, England; Edinburgh, Scotland; Lisbon,
Portugal; Dublin, Ireland; Stockholm,
Sweden; Tubingen, Germany; and Helsinki, Finland. The study investigated
the correlates of cerebral palsy (CP) in
a population sample and compared
clinical findings with information available from magnetic resonance imaging (MRI) brain studies. The aim was
that this study would help in understanding where potential preventive
strategies could be targeted.
METHODS
A population of children with CP who
were born between 1996 and 1999 were
examined at each center. They were
identified from a geographic area surrounding each center with 25 000 live
births per year. According to the usual
reported rates of CP of about 2 per 1000
live births, it was predicted that each
center would recruit approximately 100
children during the study period.
Ethical permission and written informed parental consent were obtained in all centers.
A standard clinical examination was
devised largely based on the work of
Grether et al,1 with reference to Ingram2 and Crothers and Paine.3 Cerebral palsy was defined as a group of
See also p 1650 and Patient Page.
1602
Context Magnetic resonance imaging (MRI) findings have been reported for specific clinical cerebral palsy (CP) subgroups or lesion types but not in a large population
of children with all CP subtypes. Further information about the causes of CP could
help identify preventive strategies.
Objective To investigate the correlates of CP in a population sample and compare
clinical findings with information available from MRI brain studies.
Design and Setting Cross-sectional, population-based investigative study conducted in 8 European study centers (North West London and North East London, England;
Edinburgh, Scotland; Lisbon, Portugal; Dublin, Ireland; Stockholm, Sweden; Tubingen,
Germany; and Helsinki, Finland).
Participants Five hundred eighty-five children with CP were identified who had been
born between 1996 and 1999; 431 children were clinically assessed and 351 had a
brain MRI scan.
Main Outcome Measures Standardized clinical examination results, parental questionnaire responses, MRI results, and obstetric, genetic, and metabolic data from medical records.
Results Important findings include the high rate of infections reported by mothers
during pregnancy (n=158 [39.5%]). In addition, 235 children (54%) were born at
term while 47 children (10.9%) were very preterm (28 weeks). A high rate of twins
was found, with 51 children (12%) known to be from a multiple pregnancy. Clinically, 26.2% of children had hemiplegia, 34.4% had diplegia, 18.6% had quadriplegia, 14.4% had dyskinesia, 3.9% had ataxia, and 2.6% had other types of CP. Brain
MRI scans showed that white-matter damage of immaturity, including periventricular
leukomalacia (PVL), was the most common finding (42.5%), followed by basal ganglia lesions (12.8%), cortical/subcortical lesions (9.4%), malformations (9.1%), focal
infarcts (7.4%), and miscellaneous lesions (7.1%). Only 11.7% of these children had
normal MRI findings. There were good correlations between the MRI and clinical
findings.
Conclusions These MRI findings suggest that obstetric mishaps might have occurred in a small proportion of children with CP. A systematic approach to identifying
and treating maternal infections needs to be developed. Multiple pregnancies should
be monitored closely, and the causes of infant stroke need to be investigated further
so preventive strategies can be formulated. All children with CP should have an MRI
scan to provide information on the timing and extent of the lesion.
www.jama.com
JAMA. 2006;296:1602-1608
nonprogressive motor disorders of
movement and posture due to a defect
or lesion of the developing brain.4 Children were recruited after the age of 2
years, when the diagnosis of CP can be
firmly substantiated.5
Lead clinicians involved in the study
were highly experienced in the care and
assessment of children with CP and,
JAMA, October 4, 2006Vol 296, No. 13 (Reprinted)
Author Affiliations: Department of Paediatrics,
Imperial College London, Chelsea & Westminster
Hospital, London, England (Dr Bax and Ms Tydeman);
Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden (Dr Flodmark).
Members of the European Cerebral Palsy Study are
listed at the end of this article.
Corresponding Author: Martin Bax, DM, FRCPCH,
Department of Paediatrics, Imperial College
London, Chelsea & Westminster Hospital, 369
Fulham Rd, London SW10 9NH, England
(m.bax@imperial.ac.uk).
2006 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
when possible, these clinicians examined the study children within their centers. To alleviate potential interobserver and intraobserver error, we
optimized the validity and reliability of
the examination by using a training
video highlighting points that might
present difficulties. Further training was
offered by 3 key investigators, who visited satellite centers participating in the
examination of a number of the children recruited to the study. Direct reliability studies on the coding of the
physical examination were not possible due to the complexity of the cases
and the multicenter format. Therefore, clinical characteristics were analyzed to profile the expected clinical pattern. Outliers from the usual pattern of
findings were reviewed in liaison with
the local clinician to check for any errors in coding.
An interview questionnaire with the
parents provided information about
family history and prenatal, pregnancy, and birth information. Hospital obstetric notes were sought to verify
birth data given by parents and to collect extra information on the birth and
neonatal period. The results of genetic
and metabolic investigations were recorded when performed. In total, more
than 300 items of information were requested for each child.
A very important component was the
MRI brain scan. Various articles have
reported findings from studies on children with selected types of CP, including bilateral spastic CP6 and hemiplegia,7 and types of MRI pattern, such as
periventricular leukomalacia (PVL).8
However, we are unaware of any studies reporting MRI findings in a large CP
population including all of the clinical
subtypes. Cranial MRI is a standard
diagnostic procedure safe to use in
children.
We sought MRI scans taken at 18
months of age or later. Magnetic resonance imaging reports from the participating centers were often brief and
difficult to compare, so each scan in the
study was assessed jointly by 2 study
members (O.F. and I.K.-M.) using a
standardized scoring system devel-
oped for this study. Studies were carried out in line with the local protocols for pediatric patients available in
all institutions. The sequences used
were developed in association with the
radiologists from the participating centers and were chosen for optimal information at minimum total time in the
MRI camera. Each scan was assessed
blind to both the local MRI report and
clinical findings.
Simple descriptive statistics are used
throughout the article. 2 Tests were used
to assess the statistical significance of associations between categorical variables. Case-control data were not collected because of staffing limitations.
SPSS software, version 14.0 (SPSS Inc,
Chicago, Ill) was used to analyze data.
RESULTS
In total, 585 children with CP were
identified as eligible to participate in the
study and 431 of these children were
recruited and assessed. Of the children in the study, 61.9% were male.
Of the 154 children who were not recruited, 55 parents did not consent to
the participation of their child, 21 families moved away before inclusion, 7
failed to attend appointments, 7 children died before inclusion, and 11 families could not be contacted. A further
53 children did not have a reason for
noninclusion recorded. Information
about the type of CP was available for
49 of the 154 children, and for this
group, there was no significant difference compared with the children included in the study.
Only 1 center achieved the target of
identifying 100 children with CP. Four
possible reasons for the failure to reach
the anticipated numbers are (1) failure of identification; (2) that the birth
population sample was actually smaller
than originally anticipated; (3) that centers were not accessing the entire population with CP in their area; and (4) that
there had been some genuine decline
in the rate of CP. As a consequence of
1 or more of these factors, our sample
is smaller than expected. The most
likely explanation is that there was some
underidentification of mild cases in
2006 American Medical Association. All rights reserved.
most centers because of the organization of services and methods of identification. There is no real evidence from
the study that the lower numbers reflect a decrease in the 2-per-1000 live
birth rate for CP.
Prenatal Findings
A striking finding from the mothers history was that 39.5% (158 of 400) reported an infection during the pregnancy. Specifically, 19.2% of the mothers
reported a urinary tract infection during the pregnancy and 15.5% of women
reported taking antibiotics during the
pregnancy. Two of the study team independently reviewed the infection data recorded and it was agreed that 29.6% had
a significant infection (excluding cases
of common colds, coughs, etc).
Fifty-one children (12%) were
known to be from a multiple pregnancy, with 48 from a twin pregnancy
and 3 from a triplet pregnancy. This
compares with a population rate of multiple pregnancy of about 1.5%9 In some
instances, both twins had CP and were
in the study (4 pairs), and in 13 others, their twin had previously died (7
in utero and 6 during or after birth).
Among children from multiple pregnancies, 12 (24%) were from pregnancies after infertility treatment compared with 12 (3.4%) of the singleton
pregnancies in the study.
Perinatal Findings
More than half of the children (n=235
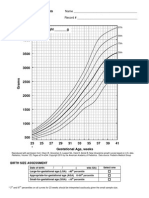
[54.5%]) were born at term. Fortyseven (10.9%) were very low-gestationalage infants (28 weeks), 69 (16%) were
born between 28 and 31 weeks, and 79
(18.3%) were born between 32 and 36
weeks of gestation (FIGURE 1). The numbers in the very low-gestational-age
group are so small that any variation in
the rates of CP in this group would not
drastically affect the overall numbers of
children with CP.
Among the study children, 19.1%
were small for gestational age (birth
weight 10th percentile), with similar rates occurring in all gestational age
groups. However the actual number of
infants who were small for gestational
(Reprinted) JAMA, October 4, 2006Vol 296, No. 13 1603
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
told about the possibility of brain damage (Pearson 42 =26.256; P.001).
Figure 1. Distribution of Gestational Age at Birth in Weeks
90
Clinical Findings
80
No. of Children
70
60
50
40
30
20
10
0
23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43
Length of Gestation, wk
Figure 2. Length of Time in Special Care Infant Unit by Gestational Age at Birth
120
No. of Children
100
Length of Time in Special
Care Infant Unit, d
0
<5
5-29
30
80
60
40
20
0
<28
(n = 45)
28-31
(n = 66)
32-36
(n = 75)
37
(n = 222)
Gestational Age at Birth, wk
Table 1. Types of Cerebral Palsy
Type of Cerebral Palsy
Hemiplegia
Diplegia
Spastic quadriplegia
Dyskinesia
Ataxia
Other
Total
No. (%)
113 (26.2)
148 (34.4)
80 (18.6)
62 (14.4)
17 (3.9)
11 (2.6)
431 (100.0)
age was higher in the term group
(n = 44) than in the preterm group
(n=31). In the group born at less than
28 weeks, 16.3% were large for gestational age, whereas children in the other
groups were just over the expected 10%
rate (10.6%-11.1%). These data were
calculated for all children in the study
using the Child Growth Foundation
growth charts.10
Emergency cesarean deliveries were
performed in 32.3% of births, whereas
44.8% of the children were born by stan1604
dard vaginal delivery. Of the rest, 7%
were delivered by planned cesarean delivery, 4.2% by Ventouse extraction, 6.1%
by forceps, and 2.3% were breech. The
delivery method was not recorded for
3.5%.
Seventy percent of children were admitted to the special care infant unit after birth. FIGURE 2 shows the length of
time spent in the special care infant unit
according to the gestational age at birth.
A total of 43.7% of children born at term
were kept in the unit for 5 or more days
and, therefore, presumed to be regarded by the attending clinician as
significantly ill in the neonatal period.
At the childrens discharge from the
special care infant unit, 47.3% of the
parents report that they were unaware
that there was any concern about their
child. The parents of children with more
severe CP were more likely to have been
JAMA, October 4, 2006Vol 296, No. 13 (Reprinted)
On completion of the physical examination, the clinician was asked to record the topographical nature of the condition (hemiplegia, diplegia, quadriplegia,
or other) and the nature of the CP (spastic, dystonic, choreoathetoid, or ataxia).
The functional severity was graded as
mild, moderate, or severe (TABLE 1).
At the time of original examination,
the children ranged in age from 12 to
91 months, with a mean age of 46
months (9 children were seen at 23
months). Where children were assessed before the age of 3 years, a request was made to reassess at a later date
to confirm the diagnosis of CP and note
any changes in presentation. Selected
variables from the physical examination are shown in TABLE 2 illustrating
some clinical differences between the
spastic CP subtypes.
During the period of data collection,
there has been further validation of the
Gross Motor Function Classification System11 in CP. To compare our coding of
functional severity to this scale, a subsample of 36 randomly selected children were retrospectively recoded to the
1 through 5 scores in the Gross Motor
Function Classification System. There
was strong correspondence between the
data (=0.583; P.001), with 1 being
mild, 2 and 3 being moderate, and 4 and
5 being severe.
Many children also have clinical findings not related to motor disorder, and
failure to include this in any definition
and classification of CP has recently been
emphasized.12 In total, 28% of the children had epilepsy; the rate was highest
(50%) among the quadriplegia group and
lowest (16%) in children with diplegia.
Communication problems were present in 58% of the total grouphighest
in the dyskinesia and quadriplegia groups
and lowest in the diplegia and hemiplegia groups.
Vision is difficult to assess clinically in young children, but it was recorded that 31% of the children with
hemiplegia, 38% of the children with
2006 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
diplegia, and 64% of those with quadriplegia had visual abnormalities of
some kind. About 7% of the children
had a hearing problem reported.
MRI Findings
Among children with clinical evaluation, 351 (81.4%) had a brain MRI scan
assessed for the study. The ages at which
the scans were taken ranged from 1 to
87 months, with a mean age of 38
months. Scans taken before the age of 18
months were only accepted for the study
if the type of pathology was obvious and
renewed imaging was not expected to
provide further information; eg, in malformations or extensive posthemorrhagic periventricular destruction. The
scans were grouped according to the primary pattern of damage (TABLE 3).
The MRI scans showed that whitematter damage of immaturity (WMDI,
including PVL) was the most common
finding (42.5%), followed by basal ganglia lesions (12.8%), cortical/subcortical lesions (9.4%), malformations (9.1%),
focal infarcts (7.4%), and miscellaneous lesions (7.1%). Normal MRI findings were present in 11.7%.
Table 2. Selected Results From Physical Examination
Type of Cerebral Palsy, No. (%)
Walking functional
Sitting functional
Abnormal hand function
Functional severity
Mild
Moderate
Severe
Involuntary movements
Scoliosis
Muscle tone
Epilepsy
Communication problems
Hemiplegia
(n = 113)
100 (89)
113 (100)
Diplegia
(n = 148)
93 (63)
125 (85)
Quadriplegia
(n = 80)
7 (9)
28 (35)
98 (87)*
80 (54)
58 (73)
70 (63)
38 (34)
3 (3)
12 (11)
48 (34)
66 (46)
29 (20)
26 (18)
6 (7)
11 (14)
63 (79)
29 (36)
4 (3)
78 (53) [Increased]
21 (16)
18 (23)
68 (85) [Increased]
39 (50)
56 (39)
71 (89)
2 (2)
96 (85) [Mixed]
29 (26)
44 (39)
dren with bilateral spastic CP, in general, those with posterior only or
posterior and middle WMDI were
found to have spastic diplegia. These
children were often able to walk and
had some communication skills. The
spastic quadriplegia group, however,
mostly had damage across all areas, and
virtually all could not walk and often
had very limited communication skills
(FIGURE 4).
White-matter damage of immaturity included PVL and periventricular hemorrhage. White-matter damage of immaturity was found in 71.3% of the
children with diplegia (n = 87), 34.1%
of those with hemiplegia (n = 31), and
35.1% of those with quadriplegia
(n=20). This type of damage is thought
to occur before about 34 weeks of gestation, although 25% of the WMDI
group were born at term.
FIGURE 3A shows 2 images through
the levels of the lateral ventricles in a
child with mild WMDI and a diagnosis of mild PVL who clinically has mild
diplegia.
In contrast, Figure 3A also shows an
image obtained at a level through the
lateral ventricles of a child with severe
WMDI and a diagnosis of severe PVL
who has quadriplegia.
When the location of the whitematter loss was investigated in the chil-
MRI Pattern
Malformation
White-matter damage
of immaturity
Focal infarct
Cortical subcortical damage
Basal ganglia damage
Miscellaneous
Normal
Total
No. (%)
32 (9.1)
149 (42.5)
26 (7.4)
33 (9.4)
45 (12.8)
25 (7.1)
41 (11.7)
351 (100)
Cortical/Subcortical Damage
Basal Ganglia Damage
White-Matter Damage
of Immaturity
Table 3. Magnetic Resonance Imaging
(MRI) Pattern Types
Basal ganglia and thalamic damage was
mainly associated with dystonic CP,
which accounted for 75.6% (n=34) of
the basal ganglia group. Occasionally,
this type of damage was seen in children with spastic quadriplegia (n=7)
and diplegia (n=4). There were no children with hemiplegia. Figure 3B shows
an image at the level of thalamus and
basal ganglia in a child with dyskinetic CP. Figure 3B also shows an image of the same child at the level of the
parietal lobes.
Focal Infarct
Focal cortical infarcts were almost exclusively related to a clinical diagnosis
of hemiplegia (n=25), with 1 child diagnosed as having quadriplegia. Among
children with hemiplegia, 27.5% were
found to have a focal infarct. Figure 3C
shows an example of a child with handdominated left hemiplegia who probably had an embolus in the right middle
cerebral artery.
2006 American Medical Association. All rights reserved.
This group included children with multicystic encephalomalacia and other cortical lesions. Eleven children (33%) had
a watershed pattern of damage. All CP
types apart from ataxia were represented in this group.
Malformations
Thirty-two children were found to have
malformations. These were most common in the hemiplegia group (n=12)
but were found across all clinical
subtypes.
Six of the malformations were
thought to be a result of specific in utero
infections, such as cytomegalovirus,
which can be identified on MRI by the
specific distribution of calcifications.13 Other types of malformation included lissencephaly, polymicrogyria,
schizencephaly, and cortical dysplasia. Figure 3D shows an image of a child
with cytomegalovirus infection who
clinically has spastic quadriplegia and
extensive dysplasia involving large parts
of both parietal lobes.
(Reprinted) JAMA, October 4, 2006Vol 296, No. 13 1605
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
Miscellaneous
Twenty-five children (7.1%) had findings on the scans that did not fit into the
aforementioned groups and were labeled
miscellaneous. They were found across
all clinical CP subtypes and were often
accompanied by high rates of other prob-
lems, such as epilepsy and visual problems. Some of these children may have
unidentified genetic abnormalities.
Normal MRI
Forty-one of the children (11.7%) had
normal MRI results. They were distrib-
uted across all clinical CP subtype
groups, although they did make up
more than half of the group with ataxia
(n = 8). Sixty-one percent of the children had mild and 17% had severe functional severity. There may be genetic defects among this group.
Figure 3. Examples of Magnetic Resonance Imaging Scans From the European Cerebral Palsy Study
A Normal
B White-Matter Damage
Mild
Severe
A, Normal T2-weighted axial image from a child aged 4 years 8 months shown for comparison. B, Left, Mild posterior periventricular white-matter damage in a child
aged 5 years 4 months with mild diplegia. Two T2-weighted axial images show increased signal (black arrowheads) in the periventricular white matter extending
posteriorly into the central white matter of the occipital lobes. Right, Severe bilateral, symmetrical periventricular leukomalacia in a child aged 5 years 6 months with
quadriplegia. A T2-weighted axial image shows almost total absence of central white matter. Gray matter extends to the ventricular wall, which is irregular. Small
amounts of white matter remain in the frontal lobes (yellow arrowheads) This tissue has abnormally high signal. White matter with abnormal signal is also seen in the
lower aspects of the corona radiata (black arrowheads).
C Basal Ganglia Lesions
T2-weighted axial images from a child aged
18 months with basal ganglia lesions and
dyskinetic cerebral palsy. The left image shows
reduction in volume and increased signal in
the posterior third of the putamen (yellow
arrowhead) and increased signal in a location
corresponding to the inferolateral nucleus of the
thalamus (black arrowhead). The lesions are
symmetrical. No other abnormalities are noted.
The right image, at the level of the parietal
lobe, shows increased signal bilaterally in
cortical structures anterior and posterior to the
central sulcus (white arrowhead). These areas
correspond to the sensory and motor cortex.
D Focal Cortical Infarct
E Cerebral Dysplasia
Coronal T2-weighted image from a child
aged 3 years 6 months with dysplasia of
both parietal lobes after cytomegalovirus
infection and spastic quadriplegia. Note
the thick cortex and irregular surface indicating polymicrogyria. Signal abnormalities were present throughout most
supratentorial white matter, some seen
here in the centrum semiovale (black
arrowhead) and in the lower temporal
lobes (yellow arrowhead).
T2-weighted axial image at the level of the
lateral ventricles from a child aged 5 years
9 months with a focal cortical infarct and
hand-dominated left hemiplegia. The right
hemicranium is significantly smaller than
the left because of reduced volume of the
right cerebral hemisphere. The right lateral
ventricle is large secondary to the small
volume of the hemisphere. There is a focal
defect corresponding to large parts of the
hemisphere supplied from the right middle
cerebral artery. The defect includes
thalamus and the basal ganglia, which are
smaller than normal. This child probably
had an embolus in the right middle
cerebral artery.
1606
JAMA, October 4, 2006Vol 296, No. 13 (Reprinted)
2006 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
Abnormal MRI
FIGURE 5 indicates the distribution of
pathologic MRI findings in 2 groups,
those born before 34 weeks of gestational age and those born at or after 34
weeks.
In summary, the MRI findings relate both to the clinical description of
the child and the severity of the condition. The timing of the lesion might
help to define which events might have
caused the CP.
COMMENT
Not only do MRI scans help reveal the
pathologic basis of the condition, but
also, the findings have strong correlations with clinical findings. This may
be useful in helping parents, clinicians, and others involved in the care
of children with CP to understand the
nature of the childrens condition and
to predict their needs in the future.
Therefore, all children with CP should
have an MRI scan.
Our study has some important implications in relationship to possible
malpractice suits. The scans can be reviewed in relationship to the pathologic findings to determine which might
have been caused by some event during the delivery.
Among infants born before 34
weeks, the great majority had whitematter damage. This is often thought
to be caused by obstetric malpractice,
but there is little evidence for this. As
pointed out by Nelson,14 changes in
obstetric practice over the last 20 years
and the development of overall neonatal care during the same period have
had little effect on the rate of CP.
Rather, predisposing factors that
include genetic factors, nutritional factors, and infections during pregnancy
and before the onset of premature
labor lead to placental damage developing throughout the pregnancy. 15
These factors predispose the infant to
an increased risk of hypoxic ischemic
episodes, leading to white-matter
damage. By the time this occurs, the
event has reached a stage at which it
cannot be affected by interventions.
This may lead to premature birth and
Figure 4. WMDI Location of White-Matter Loss Across All Areas and Bilateral Spastic
Cerebral Palsy Diagnosis
Spastic Diplegia
(n = 81)
White-Matter Loss Pattern
Spastic Quadriplegia
(n = 19)
None
Posterior Only
Posterior and Middle Only
Posterior, Middle, and Anterior
Middle Only
0
10
15
20
25
30
10
Count
15
20
25
30
Count
WMDI indicates white-matter damage of immaturity.
Figure 5. Gestational Age at Birth Before 34 Weeks or After 34 Weeks and MRI Pattern Type
<34
(n = 119)
MRI Pattern Type
Gestational Age at Birth, wk
34
(n = 232)
Normal
White-Matter Damage of Immaturity
Basal Ganglia Damage
Focal Cortical Infarcts
Cortical Subcortical Damage
Malformations
Miscellaneous
0
20
40
60
80
Percentage
100
20
40
60
80
100
Percentage
MRI indicates magnetic resonance imaging.
concerns about the infant. In other
instances, no event is noted by the
mother or her attendants, and the
child is born at term with established
white-matter damage. Interventions
around the time of either of these
events will not affect the outcome. In
their sophisticated study of stillbirths16
and early neonatal deaths17 in Scotland, Becher et al conclude that the
findings in this study support the
notion that the birth of a compromised asphyxiated encephalopathic
infant is not necessarily the result of a
mismanaged labour nor the lack of
vigilance in pregnancy.
In our study, 54 children with whitematter damage were born after 34
weeks. These, together with the 32 children with malformations, again indicative of earlier cerebral damage, would
not have been affected by perinatal processes.
Of the other groups, focal infarcts are
clearly not caused by obstetric events,
and the 25 infants in the miscellaneous group included some infec-
2006 American Medical Association. All rights reserved.
tions. In the 41 children with normal
MRI results, some infants probably had
genetic causes for their CP, but when
there is no evident lesion in the cerebral cortex, it is hard to conceive of an
obstetric cause.
This leaves 70 children with either
cortical/subcortical damage or basal
ganglia damage born after 34 weeks of
gestational age. Thus, in our study, only
19.9% of a population with CP might
be considered on the basis of their MRI
scan as having a possibility of some type
of obstetric mishap as the cause of their
brain damage.
The data on the method of delivery
in this group show that 26% of mothers had emergency cesarean deliveries,
indicating that the obstetrician was well
aware that the infant was in difficulty.
Although a proportion of these may have
had difficulties due to some obstetric inattention, many would not, and it seems
likely that the proportion of infants who
might have had damage because of obstetric malpractice within the CP population is therefore low.
(Reprinted) JAMA, October 4, 2006Vol 296, No. 13 1607
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
CLINICAL AND MRI FINDINGS IN CEREBRAL PALSY
This study has once again emphasized the great importance of infections
occurring in mothers during pregnancy. Most study centers had no access to normative data on this topic. The
closest comparable population data on
the incidence of urinary tract infection
during pregnancy that we were able to
access were through the St Marys Maternity Information System. This database has information available on 74 838
live births in the 2-year period matching our studys, collected from maternity units in the North West Thames region of London. Our finding that 19.2%
of mothers reported a urinary tract infection during the pregnancy compares
with the rate of clinically diagnosed urinary tract infection during pregnancy in
the general population of the St Marys
Maternity Information System of 2.9%.
The results appear to show a raised rate
of infection. This finding is also supported by the case-control study of Neufeld et al,18 in which maternal infection
was a risk factor for CP in both term and
preterm infants. Wu et al19 also reported a significantly increased rate of infection in children born with CP at or
near term, in which 14% of the mothers
had had chorioamnionitis compared with
4% of controls.
While there is a legitimate concern
about overprescription of antibiotics,
there should be no question that treating infections during pregnancy is a
potential area for prevention. A systematic approach to identifying and
treating maternal infections needs to
be developed, as emphasized by
Nelson.14
Twin pregnancies are another potential area for prevention of CP. Multiple pregnancies, including pregnancies after infertility treatment, should
be monitored closely, especially when
other risk factors are present.
Because of the lack of warning to parents at discharge from special care infant units about the risks of potential
problems, delay in diagnosis and referral to an appropriate service was common. These findings suggest that at
discharge from the hospital, better arrangements should be made for the fol1608
low-up of these children, particularly
as there is a possibility that further brain
damage might occur after birth from,
for example, nutritional effects.20 The
recognition of maternal and child genetic factors in thrombophilia leading
to stroke is an area in which early identification could perhaps lead to prevention.
Finally, perhaps 10% of this population of children with CP might have
unidentified genetic or metabolic disorders.
We think it is not unreasonable to assume that with increased awareness of
possible preventive measures, over the
next decade the rate of CP could be reduced substantially, thus reducing the
burden on families and saving tremendous sums of money for health services.
Author Contributions: Dr Bax had full access to all of
the data in the study and takes responsibility for
the integrity of the data and the accuracy of the data
analysis.
Study concept and design: Bax, Flodmark.
Acquisition of data: Bax, Tydeman, Flodmark.
Analysis and interpretation of data: Bax, Tydeman,
Flodmark.
Drafting of the manuscript: Bax, Flodmark.
Critical revision of the manuscript for important intellectual content: Bax, Tydeman, Flodmark.
Statistical analysis: Bax, Tydeman, Flodmark.
Obtained funding: Bax.
Administrative, technical, or material support:
Tydeman, Flodmark.
Study supervision: Bax.
Financial Disclosures: None reported.
European Cerebral Palsy Study Lead Investigators:
North West London: Martin Bax, Clare Tydeman,
Imperial College London; North East London:
Lucinda Carr, Great Ormond Street Hospital; Edinburgh: Paul Eunson, Debbie Miller, Royal Hospital
for Sick Children; Dublin: Owen Hensey, Susan
Keane, Central Remedial Clinic; Helsinki: Lennart
von Wendt, Helsinki University Central Hospital,
Teija Salokorpi, Foundation for the Rehabilitation of
Children and Young People, Mannerheim League
for Child Welfare; Tubingen: Ingeborg KragelohMann, Andrea Bevot, University Childrens Hospital; Lisbon: Maria da Graca de Campos Andrada,
Teresa Folha, Fernanda Nunes, Centro Rebilitacao
Paralisa Cerebral Calouste Gulbenkian; Stockholm:
Olof Flodmark, Hakan Svan, Gunilla Malm, Karolinska University Hospital, Eva Esscher, Sachsska
Barnsjukhuset.
Funding/Support: Ongoing funding for this study is
provided by the Castang Foundation, having been initiated by the Little Foundation.
Role of the Sponsor: The funders had no role in the
design, collection, analysis, and interpretation of data;
in the writing of the report; or in the preparation, review, or decision to submit the manuscript for publication.
Acknowledgment: We thank all those who have helped
to make this research possible, especially the children, the parents, and the families who allowed their
information to be used. We also thank the numerous
clinicians and staff involved in the care of these chil-
JAMA, October 4, 2006Vol 296, No. 13 (Reprinted)
dren who generously gave their time and efforts to
help with the study. Statistical advice was provided
by Ian Plewis, MSc, Institute of Education, University
of London. Additional advice was supplied by Paul
Eunson, MbChB, FRCPCH, Royal Hospital for Sick Children, Edinburgh, and Lucinda Carr, MD, MBChB,
FRCP, FRCPCH, Great Ormond Street Hospital for Children NHS Trust.
REFERENCES
1. Grether JK, Cummins SK, Nelson KB. The California Cerebral Palsy Project. Paediatr Perinat Epidemiol.
1992;6:339-351.
2. Ingram TTS. Paediatric Aspects of Cerebral Palsy.
London, England: E & S Livingstone Ltd; 1964.
3. Crothers B, Paine RS. The Natural History of Cerebral Palsy. London, England: Oxford University Press;
1959.
4. Bax MC. Terminology and classification of CP. Dev
Med Child Neurol. 1964;11:295-297.
5. Kuban KC, Leviton A. Cerebral palsy. N Engl J Med.
1994;330:188-195.
6. Krageloh-Mann I, Petersen D, Hagberg G, Vollmer
B, Hagberg B, Michaelis R. Bilateral spastic CPMRI
pathology and origin: analysis from a representative
series of 56 cases. Dev Med Child Neurol. 1995;
37:379-397.
7. Cioni G, Sales B, Paolicelli PB, Petacchi E, Scusa MF,
Canapicchi R. MRI and clinical characteristics of children with hemiplegic CP. Neuropediatrics. 1999;30:
249-255.
8. Hashimoto K, Hasegawa H, Kida Y, Takeuchi Y. Correlation between neuroimaging and neurological outcome in periventricular leukomalacia: diagnostic criteria.
Pediatr Int. 2001;43:240-245.
9. Dex S, Joshi H. Children of the 21st Century: From
Birth to Nine Months. Bristol, England: Policy Press;
2005.
10. Child Growth Foundation. Child Growth Foundation 1996/1 UK 9 Centile Cross Sectional Paediatric Charts. Tyne and Wear, England: Harlow Printing Ltd; 1996.
11. Palisano R, Rosenbaum P, Walter S, Russell D,
Wood E, Galuppi B. Development and reliability of a
system to classify gross motor function in children with
CP. Dev Med Child Neurol. 1997;39:214-223.
12. Bax M, Goldstein M, Rosenbaum P, Leviton A,
Paneth N. Proposed definition and classification of CP,
April 2005introduction. Dev Med Child Neurol.
2005;47:571-576.
13. Barkovich AJ, Lindan CE. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol.
1994;15:703-715.
14. Nelson KB. Can we prevent CP? N Engl J Med.
2003;349:1765-1769.
15. The Placenta and Neurodisability. London, England: Mac Keith Press; 2006.
16. Becher JC, Bell JE, Keeling JW, Liston WA, McIntosh
N, Wyatt B. The Scottish Perinatal Neuropathology
Study: clinicopathological correlation in stillbirths.
BJOG. 2006;113:310-317.
17. Becher JC, Bell JE, Keeling JW, McIntosh N, Wyatt
B. The Scottish Perinatal Neuropathology Study: clinicopathological correlation in early neonatal deaths. Arch
Dis Child Fetal Neonatal Ed. 2004;89:F399-F407.
18. Neufeld MD, Frigon C, Graham AS, Mueller BA.
Maternal infection and risk of CP in term and preterm infants. J Perinatol. 2005;25:108-113.
19. Wu YW, Escobar GJ, Grether JK, Croen LA, Greene
JD, Newman TB. Chorioamnionitis and CP in term and
near-term infants. JAMA. 2003;290:2677-2684.
20. Sullivan PB, Lambert B, Rose M, Ford-Adams M,
Johnson A, Griffiths P. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med
Child Neurol. 2000;42:674-680.
2006 American Medical Association. All rights reserved.
Downloaded From: http://jama.jamanetwork.com/ by a University of California - Los Angeles User on 04/26/2015
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Mellits and Cheek FormulaDocument5 pagesMellits and Cheek FormulafadhlialbaniNo ratings yet
- Print Jurnal 1Document1 pagePrint Jurnal 1fadhlialbaniNo ratings yet
- Altered Iron Metabolism in HIV Infection - Mechanisms, Possible Consequences, and Proposals For Management.Document1 pageAltered Iron Metabolism in HIV Infection - Mechanisms, Possible Consequences, and Proposals For Management.fadhlialbaniNo ratings yet
- Tumor Lysis Syndrome OLDocument37 pagesTumor Lysis Syndrome OLfadhlialbaniNo ratings yet
- Pathophysiology of Tethered Cord Syndrome and SimilarDocument10 pagesPathophysiology of Tethered Cord Syndrome and SimilarfadhlialbaniNo ratings yet
- 0-DM Problem Chart 2Document1 page0-DM Problem Chart 2fadhlialbaniNo ratings yet
- Pathogenesis of Encephalitis BookDocument19 pagesPathogenesis of Encephalitis BookfadhlialbaniNo ratings yet
- The Impact of Severe Congenital Heart Disease On Physical and Psychosocial Functioning in AdolescentsDocument7 pagesThe Impact of Severe Congenital Heart Disease On Physical and Psychosocial Functioning in AdolescentsfadhlialbaniNo ratings yet
- 1 - Psychological Aspects in Children and Adolescent With Congenital Heart Disease and Their ParentsDocument18 pages1 - Psychological Aspects in Children and Adolescent With Congenital Heart Disease and Their ParentsfadhlialbaniNo ratings yet
- Pediatrics-1993 - Psychosocial Risks of Chronic Health Conditions in Childhoentod and AdolescDocument5 pagesPediatrics-1993 - Psychosocial Risks of Chronic Health Conditions in Childhoentod and AdolescfadhlialbaniNo ratings yet
- Pediatrics-2008-Inattention, Hyperactivity, and School Performance in School Age Children With Complex CHDDocument11 pagesPediatrics-2008-Inattention, Hyperactivity, and School Performance in School Age Children With Complex CHDfadhlialbaniNo ratings yet
- Etiology Malnutrisi HIVDocument8 pagesEtiology Malnutrisi HIVfadhlialbaniNo ratings yet
- 7 - Quality of Life, Depressed Mood, and Self-Esteem in Adolescents WithDocument6 pages7 - Quality of Life, Depressed Mood, and Self-Esteem in Adolescents WithfadhlialbaniNo ratings yet
- Transposition of The Great Arteries: What Is It?Document3 pagesTransposition of The Great Arteries: What Is It?fadhlialbaniNo ratings yet
- Guidelines American Academy of Neurology 2011Document4 pagesGuidelines American Academy of Neurology 2011fadhlialbaniNo ratings yet
- Infantile Spasm - EmedicineDocument13 pagesInfantile Spasm - EmedicinefadhlialbaniNo ratings yet
- Infantile Spasms - Pediatric NeurolDocument1 pageInfantile Spasms - Pediatric NeurolfadhlialbaniNo ratings yet
- Growthcurves Gestational AgeDocument4 pagesGrowthcurves Gestational AgefadhlialbaniNo ratings yet
- Evaluation of Risk Factors For Severe PneumoniaDocument8 pagesEvaluation of Risk Factors For Severe PneumoniafadhlialbaniNo ratings yet
- Infantile Spasm - EmedicineDocument13 pagesInfantile Spasm - EmedicinefadhlialbaniNo ratings yet
- Treatment Guidelines For GuillainDocument15 pagesTreatment Guidelines For GuillainfadhlialbaniNo ratings yet
- WHO HIV Clinical Staging GuidelinesDocument49 pagesWHO HIV Clinical Staging Guidelinesdonovandube8235No ratings yet
- Evaluasi Anak Dengan Ambigous GenitaliaDocument12 pagesEvaluasi Anak Dengan Ambigous GenitaliafadhlialbaniNo ratings yet
- Infantile Spasms - MenkesDocument4 pagesInfantile Spasms - MenkesfadhlialbaniNo ratings yet
- Management of Elevated Serum Ferritin LevelsDocument2 pagesManagement of Elevated Serum Ferritin LevelsfadhlialbaniNo ratings yet
- Altered Iron Metabolism in HIV Infection - Mechanisms, Possible Consequences, and Proposals For Management.Document1 pageAltered Iron Metabolism in HIV Infection - Mechanisms, Possible Consequences, and Proposals For Management.fadhlialbaniNo ratings yet
- Early Developmental Delays - A Cross Validation StudyDocument5 pagesEarly Developmental Delays - A Cross Validation StudyfadhlialbaniNo ratings yet
- Royals - LordeDocument14 pagesRoyals - LordefadhlialbaniNo ratings yet
- Prevalence of Undetected Developmental Delays in Iranian ChildrenDocument10 pagesPrevalence of Undetected Developmental Delays in Iranian ChildrenfadhlialbaniNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Medical Gases: NO. Item Brand Name OriginDocument4 pagesMedical Gases: NO. Item Brand Name OriginMahmoud AnwerNo ratings yet
- Phillips LoFloDocument29 pagesPhillips LoFlokawaiiriceNo ratings yet
- Cen-Tech 63759Document8 pagesCen-Tech 63759GregNo ratings yet
- Narrative Report On Conduct of Classes-October 2021Document1 pageNarrative Report On Conduct of Classes-October 2021Jansen Roy D. JaraboNo ratings yet
- Notice: Environmental Statements Availability, Etc.: Syngenta Crop Protection, Inc., Et Al.Document8 pagesNotice: Environmental Statements Availability, Etc.: Syngenta Crop Protection, Inc., Et Al.Justia.comNo ratings yet
- 2014 EN AdvancedBootkitTechniquesOnAndroid ChenZhangqiShendiDocument66 pages2014 EN AdvancedBootkitTechniquesOnAndroid ChenZhangqiShendihombre pocilgaNo ratings yet
- Handover Paper Final 22 3 16 BJNDocument13 pagesHandover Paper Final 22 3 16 BJNsisaraaah12No ratings yet
- Polymer ProDocument25 pagesPolymer ProJeerisuda KingklangNo ratings yet
- (VOLKSWAGEN) Manual de Taller Volkswagen Jetta 1999 2006Document4 pages(VOLKSWAGEN) Manual de Taller Volkswagen Jetta 1999 2006Carlos AntonioNo ratings yet
- State/ District-Dehradun, Uttarakhand Year 2016-17Document20 pagesState/ District-Dehradun, Uttarakhand Year 2016-17jitendra rauthanNo ratings yet
- Corp Given To HemaDocument132 pagesCorp Given To HemaPaceNo ratings yet
- Dowry SystemDocument10 pagesDowry SystemBhoomejaa SKNo ratings yet
- Sikament®-4101 NS: Product Data SheetDocument2 pagesSikament®-4101 NS: Product Data SheetShihab AhamedNo ratings yet
- Asking and Showing Rooms in Hospital2Document17 pagesAsking and Showing Rooms in Hospital2Roland DelNo ratings yet
- Music Therapy: Treatment For Grade 11 Stem Students Who Suffer Stress From Basic CalculusDocument12 pagesMusic Therapy: Treatment For Grade 11 Stem Students Who Suffer Stress From Basic CalculusArvinel L. VileganoNo ratings yet
- School Administration and Supervision MAED 605Document24 pagesSchool Administration and Supervision MAED 605Jaynie Ann TapdasanNo ratings yet
- SiUS121602E Service ManualDocument222 pagesSiUS121602E Service ManualpqcrackerNo ratings yet
- Musculoskeletal 20,000 Series CPT Questions With Answers-CpcDocument16 pagesMusculoskeletal 20,000 Series CPT Questions With Answers-Cpcanchalnigam25100% (7)
- 6L45, 6L50, 6L80, 6L90: Time Tested - Industry TrustedDocument1 page6L45, 6L50, 6L80, 6L90: Time Tested - Industry TrustedCelso BidinotiNo ratings yet
- Raymond Lo - The Feng Shui of Swine FluDocument1 pageRaymond Lo - The Feng Shui of Swine Fluay2004jan100% (1)
- Unit5 TestDocument3 pagesUnit5 TestAndrea MészárosnéNo ratings yet
- ASNT QuestionsDocument3 pagesASNT Questionsshabbir626100% (1)
- Financial Risk Management (Zain Ullah)Document12 pagesFinancial Risk Management (Zain Ullah)Afaq AhmadNo ratings yet
- English CV Chis Roberta AndreeaDocument1 pageEnglish CV Chis Roberta AndreeaRoby ChisNo ratings yet
- AdEasy Adenoviral Vector SystemDocument43 pagesAdEasy Adenoviral Vector SystemDaniel PintoNo ratings yet
- UAW-FCA Hourly Contract SummaryDocument20 pagesUAW-FCA Hourly Contract SummaryClickon DetroitNo ratings yet
- FT8 - Air System - Maintenance - P&W FT8 - Solar Turbines Technical BLOGDocument3 pagesFT8 - Air System - Maintenance - P&W FT8 - Solar Turbines Technical BLOGLibyanManNo ratings yet
- High Resolution Computed Tomography of The Lungs - UpToDateDocument83 pagesHigh Resolution Computed Tomography of The Lungs - UpToDatejjjkkNo ratings yet
- LR 1350 Operating InstructionsDocument1,495 pagesLR 1350 Operating InstructionsPatrick Polujan100% (12)
- X FEDEX EIDocument13 pagesX FEDEX EINISREEN WAYANo ratings yet