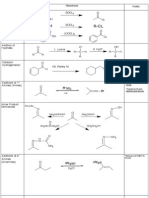
Professional Documents
Culture Documents
IUPAC Handout
Uploaded by
vivinCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
IUPAC Handout
Uploaded by
vivinCopyright:
Available Formats
Short Summary of IUPAC Nomenclature of Organic Compounds
Introduction
The purpose of the IUPAC system of nomenclature is to establish an international standard of
naming compounds to facilitate communication. The goal of the system is to give each structure
a unique and unambiguous name, and to correlate each name with a unique and unambiguous
structure.
I. Fundamental Principle
IUPAC nomenclature is based on naming a molecules longest chain of carbons connected by
single bonds, whether in a continuous chain or in a ring. All deviations, either multiple bonds or
atoms other than carbon and hydrogen, are indicated by prefixes or suffixes according to a
specific set of priorities.
II. Alkanes and Cycloalkanes
Alkanes are the family of saturated hydrocarbons, that is, molecules containing carbon and
hydrogen connected by single bonds only. These molecules can be in continuous chains (called
linear or acyclic), or in rings (called cyclic or alicyclic). The names of alkanes and cycloalkanes
are the root names of organic compounds. Beginning with the five-carbon alkane, the number of
carbons in the chain is indicated by the Greek or Latin prefix. Rings are designated by the prefix
cyclo. (In the geometrical symbols for rings, each apex represents a carbon with the number of
hydrogens required to fill its valence.)
CH4
CH3CH3
CH3CH2CH3
CH3[CH2]2CH3
CH3[CH2]3CH3
CH3[CH2]4CH3
CH3[CH2]5CH3
CH3[CH2]6CH3
CH3[CH2]7CH3
CH3[CH2]8CH3
CH3[CH2]9CH3
methane
ethane
propane
butane
pentane
hexane
heptane
octane
nonane
decane
undecane
H
CH3[CH2]10CH3
CH3[CH2]11CH3
CH3[CH2]12CH3
CH3[CH2]18CH3
CH3[CH2]19CH3
CH3[CH2]20CH3
CH3[CH2]21CH3
CH3[CH2]28CH3
CH3[CH2]29CH3
CH3[CH2]38CH3
CH3[CH2]48CH3
dodecane
tridecane
tetradecane
icosane
henicosane
docosane
tricosane
triacontane
hentriacontane
tetracontane
pentacontane
H
C
H
cyclopropane
cyclohexane
C
H
C
H
cyclobutane
cycloheptane
cyclopentane
cyclooctane
Short Summary of IUPAC Nomenclature, p. 2
III. Nomenclature of Molecules Containing Substituents and Functional Groups
A. Priorities of Substituents and Functional Groups
LISTED HERE FROM HIGHEST TO LOWEST PRIORITY, except that the substituents within
Group C have equivalent priority.
Group AFunctional Groups Indicated By Prefix Or Suffix
Family of Compound
Structure
O
Prefix
Suffix
Carboxylic Acid
C OH
O
carboxy-
-oic acid
(-carboxylic acid)
Aldehyde
C H
O
oxo(formyl)
-al
(carbaldehyde)
Ketone
C R
oxo-
-one
Alcohol
R O H
hydroxy-
-ol
Amine
R N
amino-
-amine
Group BFunctional Groups Indicated By Suffix Only
Family of Compound
Structure
Prefix
Suffix
Alkene
C C
--------
-ene
Alkyne
C C
--------
-yne
Group CSubstituents Indicated by Prefix Only
Substituent
Structure
Prefix
Suffix
Alkyl (see list below)
alkyl-
----------
Alkoxy
R O
alkoxy-
----------
Halogen
F
Cl
Br
I
fluorochlorobromoiodo-
-------------------------------------
Group C continued on next page
Short Summary of IUPAC Nomenclature, p. 3
Group CSubstituents, continued
Miscellaneous substituents and their prefixes
NO2
nitro
CH CH2
vinyl
CH2CH
allyl
CH2
phenyl
Common alkyl groupsreplace ane ending of alkane name with yl. Alternate names for
complex substituents are given in brackets.
CH3
methyl
CH3
CH
CH3
CH
CH2CH3
ethyl
CH3
isopropyl
[1-methylethyl]
CH2CH3
sec-butyl
[1-methylpropyl]
CH2CH2CH3
propyl (n-propyl)
CH2CH2CH2CH3
butyl (n-butyl)
CH2
CH3
CH
CH3
isobutyl
[2-methylpropyl]
CH3
C CH3
CH3
tert-butyl or t-butyl
[1,1-dimethylethyl]
B. Naming Substituted Alkanes and CycloalkanesGroup C Substituents Only
1. Organic compounds containing substituents from Group C are named following this sequence
of steps, as indicated on the examples below:
Step 1. Find the longest continuous carbon chain. Determine the root name for this
parent chain. In cyclic compounds, the ring is usually considered the parent chain, unless it is
attached to a longer chain of carbons; indicate a ring with the prefix cyclo before the root
name. (When there are two longest chains of equal length, use the chain with the greater number
of substituents.)
Step 2. Number the chain in the direction such that the position number of the first
substituent is the smaller number. If the first substituents from either end have the same number,
then number so that the second substituent has the smaller number, etc.
Step 3. Determine the name and position number of each substituent. (A substituent on
a nitrogen is designated with an N instead of a number; see Section III.D.1. below.)
Step 4. Indicate the number of identical groups by the prefixes di, tri, tetra, etc.
Step 5. Place the position numbers and names of the substituent groups, in alphabetical
order, before the root name. In alphabetizing, ignore prefixes like sec-, tert-, di, tri, etc., but
include iso and cyclo. Always include a position number for each substituent, regardless of
redundancies.
Short Summary of IUPAC Nomenclature, p. 4
Examples
6
1
CH3
CH
CH
CH3 CH2CH2CH3
C
4
CH
5
CH2CH2CH3
CH2CH3
Cl
Br
CH3
3-bromo-2-chloro-5-ethyl-4,4-dimethyloctane
CH3
CH
CH
CH
2 CHCH3
CH3
F
CH3
3-fluoro-4-isopropyl-2-methylheptane
H3C CHCH2CH3
1
1-sec-butyl-3-nitrocyclohexane
(numbering determined by the
alphabetical order of substituents)
6
5
3
4
NO2
C. Naming Molecules Containing Functional Groups from Group BSuffix Only
1. AlkenesFollow the same steps as for alkanes, except:
a. Number the chain of carbons that includes the C=C so that the C =C has the lower
position number, since it has a higher priority than any substituents;
b. Change ane to ene and assign a position number to the first carbon of the C =C;
c. Designate geometrical isomers with a cis,trans or E,Z prefix.
CH3
F
F
5
CH
CH CH
CH2
CH3
4,4-difluoro-3-methylbut-1-ene
CH
CH2
CH3
1,1-difluoro-2-methylbuta-1,3-diene
4
2
5-methylcyclopenta1,3-diene
Special case: When the chain cannot include the C=C, a substituent name is used.
CH
CH2
3-vinylcyclohex-1-ene
2. AlkynesFollow the same steps as for alkanes, except:
a. Number the chain of carbons that includes the CtC so that the functional group has the
lower position number;
b. Change ane to yne and assign a position number to the first carbon of the CtC.
Note: The Group B functional groups (alkene and alkyne) are considered to have equal priority:
in a molecule with both a double and a triple bond, whichever is closer to the end of the chain
determines the direction of numbering. In the case where each would have the same position
number, the double bond takes the lower number. In the name, ene comes before yne
because of alphabetization. See examples on next page.
Short Summary of IUPAC Nomenclature, p. 5
H
F
1
CH
CH
CH
HC
F
CH3
4,4-difluoro-3-methylbut-1-yne
C C CHCH3 HC C CH2 CH CH2
pent-3-en-1-yne
pent-1-en-4-yne
("yne" closer to end ("ene" and "yne" have equal
of chain)
priority unless they have the
same position number, when
"ene" takes the lower number)
(Notes: 1. An e is dropped if the letter following it is a vowel: pent-3-en-1-yne , not 3pent-3-ene-1-yne. 2. An a is added if inclusion of di, tri, etc., would put two consonants
consecutively: buta-1,3-diene, not but-1,3-diene.)
D. Naming Molecules Containing Functional Groups from Group APrefix or Suffix
In naming molecules containing one or more of the functional groups in Group A, the group of
highest priority is indicated by suffix; the others are indicated by prefix, with priority equivalent
to any other substituents. The table in Section III.A. defines the priorities; they are discussed
below in order of increasing priority.
Now that the functional groups and substituents from Groups A, B, and C have been described, a
modified set of steps for naming organic compounds can be applied to all simple structures:
Step 1. Find the highest priority functional group. Determine and name the longest
continuous carbon chain that includes this group.
Step 2. Number the chain so that the highest priority functional group is assigned the
lower number.
Step 3. If the carbon chain includes multiple bonds (Group B), replace ane with ene
for an alkene or yne for an alkyne. Designate the position of the multiple bond with the
number of the first carbon of the multiple bond.
Step 4. If the molecule includes Group A functional groups, replace the last e with the
suffix of the highest priority functional group, and include its position number.
Step 5. Indicate all Group C substituents, and Group A functional groups of lower
priority, with a prefix. Place the prefixes, with appropriate position numbers, in alphabetical
order before the root name.
1. Amines: prefix: amino-; suffix: -aminesubstituents on nitrogen denoted by N
CH3O
CH3CH2CH2
NH2
NH2
propan-1-amine
CH3CH2
CH2
3-methoxycyclohexan-1-amine
("1" is optional in this case)
CH
CH2CH3
CHCH3
N,N-diethylbut-3-en-2-amine
Short Summary of IUPAC Nomenclature, p. 6
2. Alcohols: prefix: hydroxy-; suffix: -ol
OH
OH
CH3CH2
OH
H3C
ethanol
CH
CH CH2
but-3-en-2-ol
NH2
2-aminocyclobutan-1-ol
("1" is optional in this case)
3. Ketones: prefix: oxo-; suffix: -one
O
O
CH3
CH C
CH3
OH
3-hydroxybutan-2-one
CH3
H3C
CH3
CH2 C
CH2
4-(N,N-dimethylamino)pent-4-en-2-one
cyclohex-3-en-1-one
("1" is optional in this case)
4. Aldehydes: prefix: oxo-, or formyl- (O=CH-); suffix: -al (abbreviation: CHO).
An aldehyde can only be on carbon 1, so the 1 is generally omitted from the name.
O
HCH
CH3
OH
CH
CH2 CH
CH
CH
CH3CCH2CH2
CH
methanal;
ethanal;
4-hydroxybut-2-enal
4-oxopentanal
formaldehyde acetaldehyde
Special case: When the chain cannot include the carbon of the CHO, the suffix carbaldehyde
is used:
O
CH
cyclohexanecarbaldehyde
5. Carboxylic Acids: prefix: carboxy-; suffix: -oic acid (abbreviation: COOH).
A carboxylic acid can only be on carbon 1, so the 1 is generally omitted from the name.
O
HC OH
CH3C OH
methanoic acid; ethanoic acid;
acetic acid
formic acid
O
CH2 CH COH
CH3
HC
COOH
NH2
CH3
2-amino-3-phenylpropanoic acid 2,2-dimethyl-3,4dioxobutanoic acid
(Note: Chemists traditionally use, and IUPAC accepts, the names formic acid and acetic
acid in place of methanoic acid and ethanoic acid.)
Special case: When the chain numbering cannot include the carbon of the COOH, the suffix
carboxylic acid is used. See example on next page.
Short Summary of IUPAC Nomenclature, p. 7
CHO
2
1
4
COOH
2-formyl-4-oxocyclohexanecarboxylic acid
("formyl" is used to indicate an aldehyde as
a substituent when its carbon cannot be in
the chain numbering)
E. Naming Carboxylic Acid Derivatives
The six common groups derived from carboxylic acids are salts, anhydrides, esters, acyl halides,
amides, and nitriles. Salts and esters are most important.
1. Salts of Carboxylic Acids
Salts are named with cation first, followed by the anion name of the carboxylic acid, where ic
acid is replaced by ate :
acetic acid
butanoic acid
cyclohexanecarboxylic acid
becomes
becomes
becomes
acetate
butanoate
cyclohexanecarboxylate
2. Esters
Esters are named as organic salts that is, the alkyl name comes first, followed by the name of
the carboxylate anion. (common abbreviation: COOR)
carboxylate
alkyl
CH3 O
CH3
R C O
R
H3C C O CH2CH3 H3C C
C O CHCH3
"alkanoate" "alkyl"
ethyl acetate
CH3
"alkyl alkanoate"
isopropyl 2,2-dimethylpropanoate
O
CH2
CH C O CH
vinyl prop-2-enoate
CH2
HO
CH2COO
O
C
OCH3
cyclohexyl 2-phenylacetate
methyl 3-hydroxycyclopentanecarboxylate
IV. Nomenclature of Aromatic Compounds
Aromatic compounds are those derived from benzene and similar ring systems. As with
aliphatic nomenclature described above, the process is: determining the root name of the parent
ring; determining priority, name, and position number of substituents; and assembling the name
in alphabetical order. Functional group priorities are the same in aliphatic and aromatic
nomenclature.
Short Summary of IUPAC Nomenclature, p. 8
A. Common Parent Ring Systems
8
1
2
or
6
5
benzene
3
5
naphthalene
10
anthracene
B. Monosubstituted Benzenes
1. Most substituents keep their designation, followed by the word benzene:
Cl
NO2
CH2CH3
chlorobenzene
nitrobenzene
ethylbenzene
2. Some common substituents change the root name of the ring. IUPAC accepts these as root
names, listed here in decreasing priority:
COOH
benzoic
acid
SO3H
CHO
benzenebenzaldehyde
sulfonic acid
OH
NH2
phenol
aniline
OCH3
anisole
C. Disubstituted Benzenes
1. Designation of substitutiononly three possibilities:
X
X
CH3
toluene
Y
Y
common:
IUPAC:
ortho1,2-
Y
para1,4-
meta1,3-
2. Naming disubstituted benzenesPriorities determine root name and substituents
Br
COOH
HO
NH2
OCH3
Br
CHO
3-aminobenzoic acid
1,4-dibromobenzene
2-methoxybenzaldehyde
CH3
3-methylphenol
Short Summary of IUPAC Nomenclature, p. 9
D. Polysubstituted Benzenes
CH3
Cl
HN
CH3
O2N
COOCH2CH3
NO2
Cl
OH
NO2
3,4-dichloro-N-methylaniline
NH2
2,4,6-trinitrotoluene ethyl 4-amino-3-hydroxybenzoate
(TNT)
E. Aromatic Ketones
A special group of aromatic compounds are ketones where the carbonyl is attached to at least one
benzene ring. Such compounds are named as phenones, the prefix depending on the size and
nature of the group on the other side of the carbonyl. These are the common examples:
O
O
C CH3
C CH2CH3
acetophenone
O
C CH2CH2CH3
butyrophenone
propiophenone
O
C
benzophenone
Courtesy of Dr. Jan Simek, California Polytechnic State University at San Luis Obispo
You might also like
- Cheltenham Girls 2019 Trial PaperDocument37 pagesCheltenham Girls 2019 Trial PaperYuanfeng WeiNo ratings yet
- Revision of IsomerismDocument20 pagesRevision of IsomerismAjnish GuptaNo ratings yet
- 1e Aldehyde & KetoneDocument48 pages1e Aldehyde & KetoneJonathan Wyatt100% (1)
- Chapter 12 PDFDocument35 pagesChapter 12 PDFTamara M KaramNo ratings yet
- IsomerismDocument62 pagesIsomerismsubesinghNo ratings yet
- Equilibrium Constant KC ExplainedDocument49 pagesEquilibrium Constant KC ExplainedJagmohan SinghNo ratings yet
- Chemistry Module 3Document14 pagesChemistry Module 3MASHNo ratings yet
- Naming of Alkane Alkene AlkyneDocument50 pagesNaming of Alkane Alkene AlkyneEdson BilocuraNo ratings yet
- Naming of Alkanes, Alkenes and AlkynesDocument34 pagesNaming of Alkanes, Alkenes and AlkynesArt Caresosa-FernandoNo ratings yet
- 6.2 Equilibrium Constants 1718Document28 pages6.2 Equilibrium Constants 1718Ainaa NajwaaNo ratings yet
- Ionic Equilibrium Lecture NotesDocument40 pagesIonic Equilibrium Lecture Noteskaushik2470% (1)
- Carbonyl Condensation ReactionsDocument41 pagesCarbonyl Condensation ReactionsVladislav PapperNo ratings yet
- Organic Chemistry Notes For Technical SchoolsDocument44 pagesOrganic Chemistry Notes For Technical SchoolsSheambom NelsonNo ratings yet
- Acid BaseDocument28 pagesAcid BaseDwi Fitriyana PutriNo ratings yet
- 11.alcohol, Phenol & Ethers Colour BookletDocument84 pages11.alcohol, Phenol & Ethers Colour BookletVishal Malik100% (1)
- States of Matter and Changes of StateDocument96 pagesStates of Matter and Changes of StateKay chombaNo ratings yet
- Organic Chemistry - 103 - Lecture 1Document41 pagesOrganic Chemistry - 103 - Lecture 1Abdus SubhanNo ratings yet
- Experiment 4Document7 pagesExperiment 4Pratik PatelNo ratings yet
- Alkanes NotesDocument30 pagesAlkanes NotesSabina SabaNo ratings yet
- Ch 16 Aromatic Compounds NotesDocument10 pagesCh 16 Aromatic Compounds NotesVirendra Singh Rajput100% (1)
- Introduction To Carbon CompoundDocument35 pagesIntroduction To Carbon CompoundMohd NorihwanNo ratings yet
- Matriculation Chemistry (Hydrocarbon) Part 2 AlkaneDocument30 pagesMatriculation Chemistry (Hydrocarbon) Part 2 AlkaneridwanNo ratings yet
- Organic Chemistry 2 - Syllabus - USTHDocument3 pagesOrganic Chemistry 2 - Syllabus - USTHMinh MinhNo ratings yet
- Chapter 10 PDFDocument10 pagesChapter 10 PDFKelsi Kyla PeraltaNo ratings yet
- CH 13 Titrations in Analytical ChemistryDocument14 pagesCH 13 Titrations in Analytical ChemistryHenrique CostaNo ratings yet
- Shapes of Molecules and Ions PDFDocument9 pagesShapes of Molecules and Ions PDFMagenta SparklegemNo ratings yet
- Introduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)Document24 pagesIntroduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)ajju_208180% (5)
- Chemistry Form 4 Definition ListDocument3 pagesChemistry Form 4 Definition ListAliif IsmailNo ratings yet
- Chapter 1 - Introduction To Organic ChemistryDocument102 pagesChapter 1 - Introduction To Organic ChemistryMELVINDO JACOBNo ratings yet
- Unit 4 Organic Chemistry ReactionsDocument6 pagesUnit 4 Organic Chemistry ReactionsRobbing_Hood100% (1)
- Topic 2 Atoms, Elements and CompoundsDocument79 pagesTopic 2 Atoms, Elements and CompoundsNorazian Binti TaatNo ratings yet
- Organic ChemistryDocument26 pagesOrganic Chemistryapi-379837460% (5)
- Alkanes and Alkenes: Properties, Reactions and UsesDocument3 pagesAlkanes and Alkenes: Properties, Reactions and UseskinaNo ratings yet
- Molecularity of Molecules in ChemistryDocument13 pagesMolecularity of Molecules in ChemistryAhmed HassanNo ratings yet
- Amines QuestionsDocument5 pagesAmines Questionsamal gainNo ratings yet
- IUPAC names hydrocarbonsDocument28 pagesIUPAC names hydrocarbonsRigen Alam100% (1)
- Organic Chemistry-I Reactive Intermeditate - Carbo Cation, Carbanion, Free Radicals and Carbenes.Document29 pagesOrganic Chemistry-I Reactive Intermeditate - Carbo Cation, Carbanion, Free Radicals and Carbenes.boopathi_chemist3628No ratings yet
- Hydroxyl Compounds Tutorial 6 Key ConceptsDocument21 pagesHydroxyl Compounds Tutorial 6 Key ConceptsJohnNo ratings yet
- IUPAC Nomenclature of Organic Chemistry: Basic PrinciplesDocument17 pagesIUPAC Nomenclature of Organic Chemistry: Basic PrinciplesSUBHENDU5174124No ratings yet
- Carbonyl Compounds: Aldehydes and KetonesDocument9 pagesCarbonyl Compounds: Aldehydes and KetonesCamille AdleNo ratings yet
- Chapter 11 Introduction To Organic Chemistry: HydrocarbonsDocument14 pagesChapter 11 Introduction To Organic Chemistry: HydrocarbonsKatie-Nicole ChantalNo ratings yet
- Name Reactions-II PDFDocument32 pagesName Reactions-II PDFEstanislao Amadeo Avogadro100% (1)
- Organic Chemistry Syllabus Final VersionDocument5 pagesOrganic Chemistry Syllabus Final VersionYseemaz AzeeraNo ratings yet
- Organic Chemistry NotesDocument24 pagesOrganic Chemistry NotesSweatNo ratings yet
- Orgo Reaction SheetDocument9 pagesOrgo Reaction SheetKyle Broflovski100% (1)
- Organic Chemistry (Alkane, Alkene and Alkyne)Document7 pagesOrganic Chemistry (Alkane, Alkene and Alkyne)Dazell Varron100% (1)
- Worksheet-Nernst Equation PDFDocument4 pagesWorksheet-Nernst Equation PDFLedd SleddNo ratings yet
- Intro To Organic Reactions CHM457Document73 pagesIntro To Organic Reactions CHM457Zafrel ZaffNo ratings yet
- Reactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionDocument51 pagesReactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionmacybnzNo ratings yet
- Alkanes and Organic CompoundsDocument38 pagesAlkanes and Organic CompoundsMary Darice WongNo ratings yet
- 17 Petrucci10e CSMDocument104 pages17 Petrucci10e CSMElah PalaganasNo ratings yet
- Topic 10 Organic Chemistry 10.1 To 10.2 20.1 To 20.3Document120 pagesTopic 10 Organic Chemistry 10.1 To 10.2 20.1 To 20.3Supriyaa ChordiaNo ratings yet
- IUPAC Nomenclature 1 5Document5 pagesIUPAC Nomenclature 1 5Ömer KhanNo ratings yet
- Nomenclature of Organic Compounds-3Document32 pagesNomenclature of Organic Compounds-3Muhammad ArhamNo ratings yet
- Carboxylic acid derivatives lectureDocument19 pagesCarboxylic acid derivatives lectureMustafa SaßerNo ratings yet
- Chapter 7Document30 pagesChapter 7c4.arsyadNo ratings yet
- Simple IUPAC NomenclatureDocument15 pagesSimple IUPAC Nomenclatureapi-3757218100% (6)
- Chapter 10 Efliza 2021Document37 pagesChapter 10 Efliza 2021NURIN SOFIYA BT ZAKARIA / UPMNo ratings yet
- CC One ShotDocument29 pagesCC One Shotbobbytext8904No ratings yet
- Anaranjado de MetiloDocument2 pagesAnaranjado de MetiloLuis GbNo ratings yet
- Nandesari DataDocument47 pagesNandesari DataVaibhavNo ratings yet
- Chemistry A2Document12 pagesChemistry A2Asghar AbbasNo ratings yet
- Amino Acid Structure, Properties and FunctionsDocument39 pagesAmino Acid Structure, Properties and FunctionsGirum SolomonNo ratings yet
- Dyeability of Polyester and Polyamide Fabrics Employing Citric AcidDocument14 pagesDyeability of Polyester and Polyamide Fabrics Employing Citric AcidAmi SaNo ratings yet
- Hydrocarbons Chapter 13 ReviewDocument70 pagesHydrocarbons Chapter 13 ReviewGururaj Vasisth100% (3)
- Titration Calculations: Calculating A ConcentrationDocument3 pagesTitration Calculations: Calculating A ConcentrationMohamed MaherNo ratings yet
- Alkanes Worksheet and Key02!25!09Document8 pagesAlkanes Worksheet and Key02!25!09Mr-Mohamed AliNo ratings yet
- 8 - Citric-Acid-Monohydrate EP 10Document2 pages8 - Citric-Acid-Monohydrate EP 10asmae.labindusNo ratings yet
- Athnasios A. K. - Ullmann's Encyclopedia of Industrial Chemistry (2005)Document1,169 pagesAthnasios A. K. - Ullmann's Encyclopedia of Industrial Chemistry (2005)Shelian Putri100% (1)
- The Twentieth Century English-Hindi Dictionary Containing Terms Relating to Mineralogical and Metallurgical Industries, Chemical Industries, Sugar Industry, Textile Industry, Dairy Industry, Silk Industry,Document214 pagesThe Twentieth Century English-Hindi Dictionary Containing Terms Relating to Mineralogical and Metallurgical Industries, Chemical Industries, Sugar Industry, Textile Industry, Dairy Industry, Silk Industry,Animesh_Singh1No ratings yet
- Experiment - Salt Analysis Ammonium AcetateDocument1 pageExperiment - Salt Analysis Ammonium AcetateprafullNo ratings yet
- A Review - Dye Yielding Sources and Their ImportanceDocument8 pagesA Review - Dye Yielding Sources and Their ImportanceAnne CalyxNo ratings yet
- Observe Effects of Diffusion and OsmosisDocument24 pagesObserve Effects of Diffusion and OsmosisKelwane Mc LeanNo ratings yet
- Prelab 6 Cyclohexyl ChlorideDocument5 pagesPrelab 6 Cyclohexyl ChlorideAndrea RonquilloNo ratings yet
- Titration 3 Oxalic AcidDocument5 pagesTitration 3 Oxalic AcidtisyadhruvNo ratings yet
- 1 Metallurgy SB 2023Document39 pages1 Metallurgy SB 2023Bella CakieNo ratings yet
- Ion Exchange Treatment of Drinking Water: WWW - Des.nh - Gov/organization/commissioner/pip/factsheets/dwgb/index - HTMDocument6 pagesIon Exchange Treatment of Drinking Water: WWW - Des.nh - Gov/organization/commissioner/pip/factsheets/dwgb/index - HTMmyco samNo ratings yet
- Module 7 & 8: Carbohydrates: Name: Group No.: 6Document10 pagesModule 7 & 8: Carbohydrates: Name: Group No.: 6Ma. Lara Micaela LegaspiNo ratings yet
- Unregularized Mutagenic Effects of Azo DyesDocument13 pagesUnregularized Mutagenic Effects of Azo DyesDanish IqbalNo ratings yet
- Organic Compounds in Everyday LifeDocument2 pagesOrganic Compounds in Everyday LifeVidgezxc LoriaNo ratings yet
- All about optical brightening agentsDocument13 pagesAll about optical brightening agentssusheel deoraNo ratings yet
- Alkohol FenolDocument5 pagesAlkohol FenolAnnisaa' Cahya SugiartiNo ratings yet
- Synthetic Organic Chemicals - PDF RoomDocument340 pagesSynthetic Organic Chemicals - PDF RoomM. Shehryar KhanNo ratings yet
- Phase Transfer CatalystDocument6 pagesPhase Transfer CatalystPMH100% (3)
- Coordination Compounds IDocument58 pagesCoordination Compounds IAli Muhammad Kamba60% (5)
- Hydrometallurgy Del Cu PDFDocument528 pagesHydrometallurgy Del Cu PDFAnonymous FfIxH2o9100% (1)
- Deactivation of Hydro Processing CatalystsDocument115 pagesDeactivation of Hydro Processing Catalystsphelphos1No ratings yet
- Plasticizers in Transdermal Delivery SystemsDocument24 pagesPlasticizers in Transdermal Delivery SystemsCiontu ValentinNo ratings yet
- Assignment F22 1Document15 pagesAssignment F22 1linkeyue330No ratings yet
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Napoleon's Buttons: 17 Molecules That Changed HistoryFrom EverandNapoleon's Buttons: 17 Molecules That Changed HistoryRating: 4 out of 5 stars4/5 (25)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The History of Chemistry (Vol.1&2): Complete EditionFrom EverandThe History of Chemistry (Vol.1&2): Complete EditionRating: 1 out of 5 stars1/5 (1)