Professional Documents
Culture Documents
UOP Maximize Propylene From Your FCC Unit Paper
Uploaded by
Tato FloresCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
UOP Maximize Propylene From Your FCC Unit Paper
Uploaded by
Tato FloresCopyright:
Available Formats
SpecialReport
Refining developments
Originally appeared in:
September 2011, pgs 91-95.
Used with permission.
Maximize propylene
from your FCC unit
Innovative use of catalyst and operating conditions
increases on-purpose olefin production
J. Knight and R. Mehlberg, UOP LLC, A Honeywell Co., Des Plaines, Illinois
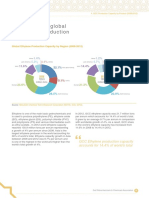
propylene demand and supply contribution by source. Although
conventional-fuels-based FCC yields approximately 4 wt%6
wt% of propylene; operating conditions, catalyst system and
technology via revamps can increase propylene yields by as much
as 5 wt%. In addition, new technologies are now available for
both revamp and new unit applications and that enable propylene
yields over 20 wt%. The fundamental question remains, what is
the most economic propylene production solution from an FCC
that takes into account the following:
10
Demand
Ethanol
MTBE
CAFE
2000
2005
2010
2015
2020
2025
Data Source: Global Petroleum Market Outlook 2011, Purvin and Gertz
Fig. 1
Gasoline demand growth and ethanol contribution.
120
New FCC
Based C3=
FCC on-purpose
Refinery/other (incl. FCC)
Steam cracker
100
80
60
40
20
Data Source: CMAI 2010 World Light Olefins Analysis (WLOA)
Fig. 2
Expected worldwide propylene demand.
HYDROCARBONPROCESSING September 2011
2020
2019
2017
2018
2015
2016
2013
2014
2011
2012
2009
2010
2007
0
2008
outlook in global propylene demand will outstrip co-production
from available ethylene crackers, FCC units and other sources.
This anticipated supply gap is expected to be filled through additional on-purpose propylene production from FCC units and
other on-purpose cracking solutions. Fig. 2 provides the expected
Prices
2005
Worldwide propylene. In contrast to gasoline, the 10-year
Imports
Production-exp.
Ethanol
Propylene demand, million metric tpy
In general, demand for clean transportation fuels will outpace
demand growth for other refined products; this is an encouraging projection for the conversion-based refiners. Conversely,
data indicates that the outlook for US gasoline demand to 2020
shows a lower overall demand and a gradual decline (Fig. 1). This
demand behavior can be explained by a number of factors:
A sharp price increases since 2004 causing the first wave of
demand destruction
The post recession recovery for US gasoline demand in 2010
is nearly 8% lower than its peak in 2004. It is expected to decline
to less than 0.5%/yr.
Ethanol blending is displacing petroleum-derived gasoline.
From 20002009, ethanol usage as a gasoline blendstock steadily
increased to its present average level of 4.5 vol%.
The Energy Independence and Security Act (EISA) was
signed into law by President Bush in December 2007. The EISA
mandates, among other items, transportation efficiency improvements that include:
By 2016, the CAFE (corporate average fuel economy)
standards for new light duty vehicles will increase by 40%.
The Renewable Fuels Standard (RFS) calls for a total of
36 billion gallons/yr of renewable fuel by 2022.
Propagation of hybrid power train technologies.
Demand, million bpd
Market Overview
2006
luidized catalytic cracking (FCC) technology was developed
to increase gasoline production derived from crude-derived
vacuum gasoil (VGO) and, in some cases, atmospheric resids.
T his continues to be the primary objective. According to a Purvin
and Gertz study, as of 2010, cracking-based conversion accounts
for approximately 50% of the worlds refining capacity with an
additional 10%15% for the North American refining market. As
fuels market needs to evolve, FCC technologies are being repurposed to produce high-grade petrochemical feedstocks along with
transportation fuels. This article investigates FCC evolving operations to meet future market needs.
SpecialReport
Refining developments
Propylene yield, wt%FF
24
Pure play C3=
producers
18
12.5
12
feed
11.8
feed
6
0
Base
FCC
Fig. 3
Increasing FCC processing severity
New
technology
FCC propylene yield as a function of feed and reactor
severity.
Gasoil
FCC catalyst
Naphtha olefins
ZSM-5
-CC-
FCC catalyst
+H
C3=
X
C2=
Fig. 4
C4=
C5=
X
FCC catalyst
+H
Paraffins
Naphthenes
Aromatics
FCC reaction pathways to produce olefins.
1. Expected fuels demand. Propylene production from an
FCC has a direct negative impact on the quality of fuel products
produced, in particular FCC naphtha. The impact is measured
in terms of reduced naphtha yield and a shift in its molecular
composition,
2. The highest propylene yield achievable given feedstock
quality. The total potential for propylene from a particular FCC
feed is determined largely by its hydrogen content.
CHEMISTRY OF PROPYLENE PRODUCTION
As the operating (reactor) severity of the FCC is increased, liquefied petroleum gas (LPG) and propylene production increases.
Propylene production is accomplished through the cracking of olefinic naphtha to lower molecular weight olefins. Fig. 3 shows that
there is a broad, continuous range of propylene yield from FCC
technology for a gasoline operation at 4 wt%6 wt-% propylene
to a petrochemical operating mode exceeding 20wt% propylene
yield. This figure summarizes the general relationship between
yields for propylene at increasing operating severities for different
quality feedstocks.
Operating variables and chemical principles. Propylene
production from an FCC unit is framed by several factors that
when combined with licensed technology provide the means for
propylene/petrochemical modes of operation. These factors include:
FCC feed quality is the most critical parameter in determining
propylene production potential. There is a strong positive correlation between the FCC feed hydrogen content and propylene
yield. Feeds that are richer in hydrogen are capable of producing
more propylene largely due to increased feedstock conversion.
Furthermore, this potential for propylene is harnessed by the FCC
technology and process conditions. The continuum of propylene
yield as a function of technology and operating conditions is
conveyed in Fig. 3.
The feed hydrogen content is responsible for the width of the
band in Fig. 3. Refiners with upstream feed-pretreating capability
may be able to leverage this condition to further extend propylene
production capacity.
Unit conversion drives propylene yield and is closely related
to feed hydrogen content. Propylene yield increases nearly linearly
with conversion. A conversion increase is typically accomplished
via rising reactor temperature and catalyst-to-oil ratio for a given
feed and catalyst system.
In the catalyst system, two elements that must be considered
are the formulation of cracking catalyst USY zeolite and the level
of pentasil within the system. As shown in Fig. 4, the first function
of the catalyst system is VGO conversion to naphtha olefins. The
second function of a high-propylene catalyst system is a pentasil
function to rapidly crack the naphtha olefins into light olefins.
Pentasil containing additives (ZSM-5) can increase propylene
yield by a factor of two to three depending on the pentasil quantity. While ethylene co-production is generally understood, this
is a kinetically controlled process that must be moderated for
propylene maximization.
An additional consideration is the formulation parameters that
mitigate hydrogen transfer reactions of olefins to paraffins and
aromatics. The level of USY zeolite and its rare earth exchange
directly impacts hydrogen transfer reactions.
The pentasil and the cracking catalyst must function cooperatively as a system, and the pentasil quantity must be balanced
with the characteristics of the cracking catalyst.
Reactor hydrocarbon partial pressure can shift the FCC
reaction equilibrium to favor low molecular weight olefins. The
reduced partial pressure is achieved collectively with lowering
operating pressure and the addition of reactor steam. An FCC
utilizing new propylene technology fundamentals can operate
at much lower partial pressure than a typical FCC while producing 50% higher propylene yields for the same feed, catalyst
and conversion.
A major engineering study was conducted to determine the
relationship between propylene yield and plant cost. Larger
equipment is required to process the increased molar flow and
to ensure the larger vapor volumes resulting from the higher
light-ends yields. These principles can also be applied to existing
units, though a partial pressure floor is imposed by throughput
and equipment design. Results of this study are data, as shown
in Fig. 5. The data describe the relationship between propylene
yield and relative plant cost for a reduction in partial pressure.
Equilibrium. Pilot plant and commercial studies have determined that commercial-scale FCC operation at high severity
and with a ZSM-5 enhanced catalyst system produces light
olefins in an equilibrium distribution. This equilibrium limit
was determined by extensive monitoring of high propylene FCC
units, bench-scale pure compound studies and circulating riser
pilot-plant testing. Fig. 6 shows the distribution of light olefins
by carbon number in the riser effluent (red bars).
HYDROCARBONPROCESSING September 2011
SpecialReport
Refining developments
COMMERCIAL APPLICATION
Considering that a base fuels FCC operation produces exreactor propylene yields in the range of 4wt%6 wt%, we examine
the requirements to further increase reactor propylene yield. Three
categories can be defined that are characterized by the extent of
scope, with each requiring additional capital and operational
expense. For simplicity, these brackets do not consider the refiners
base operation and configuration in terms of open capacity or
propylene specific equipment. Considerations for open and available capacity include, but are not limited to:
Reactor section to address the additional molar flow
Regeneration section to address additional coke make due
to higher operating severity
Recovery section to address change in vapor liquid distribution and propylene recovery
Treating section to meet the polymer-grade propylene product requirements.
Incremental reactor propylene yield above the Base Case is
bracketed according to these factors:
a. 3% to 5%Achieved through modifications to the catalyst system (use of shape selective ZSM-5 additive, modifying the
cracking catalyst formulation) and an increase in reactor temperature. Typically, such increases require only modest changes to the
recovery section and a metallurgical check to determine capacity
to accommodate the reactor temperature increase.
5x
3x
2x
1x
Relative plant cost
C3= yield, wt% FF
C3= yield
Plant cost
1
1
Fig. 5
Partial pressure
Plant cost as a function for propylene yield.
56
Riser outlet partial pressure, kPa
Equilibrium calculations indicate that the light olefin products
distribute by molecular weight and that this distribution is governed by thermodynamic equilibrium. Superimposing the distribution of light olefins from an equilibrium model calculation (blue
bars) on the data from an FCC operating in enhanced LPG operating mode shows the C3C5 olefins to be nearly in equilibrium.
This observation suggests that the reactions producing light olefins
may be controlled by equilibrium. To achieve propylene yields
significantly in excess of 12 wt% for an average FCC feedstock,
we must consider technology that acts to shift the equilibrium.
To test the hypothesis of equilibrium-limited propylene, C3
C5 olefins were independently added to a VGO feed and processed in a circulating pilot plant with a high ZSM-5 equilibrium
catalyst. The results validated the equilibrium hypothesis. When
propylene is added to the VGO feed, the net propylene yield
decreased from over 10 wt% to less than 4 wt% while at the same
time, the net yield of C4C8 olefins increased measurably. The
independent addition of 1-butene decreased the net yield of total
butenes and increased the net yield of propylene. The testing also
showed that ethylene is also influenced by equilibrium, although
not to the same extent, as residence time has the dominating
effect. A comparison of the equilibrium calculations against the
commercial unit data confirms that an equilibrium model of
FCC reactions can be used as an accurate predictive tool. In short,
reactions producing light olefins are controlled by an equilibrium
mechanism and the thermodynamic equilibrium limits propylene
production from the FCC.
Based on this evaluation, a three-year comprehensive pilot plant,
modeling and commercial benchmarking program were used to
develop two models to handle the full spectrum of low to high-propylene FCC operationsthe VGO and resid model and the olefin
re-cracking model. The former augments the traditional yield and
heat balance calculation with the effects of ZSM-5 interactions with
reaction variables, while the latter describes riser cracking of light
naphtha C4C10 olefins over ZSM-5 modified catalyst systems.
Equilibrium
49
42
Equilibrium
World-scale LPG + gasoline
Commercial
High ZSM-5
Operation
35
28
High ZSM-5
Appears to
Equilibrate
C3= to C5=
21
14
7
0
Fig. 6
C2=
C3=
C4=
C5=
C6=
C7=
C8=
C9=
C10=
FCC light olefin equilibrium distribution.
b. 5% to 9%Same as list in item a, along with reduced
hydrocarbon partial pressure. This is achieved by lowering the
reactor pressure and/or adding reactor steam. Implementation
may require modifications to enable additional wet-gas-compression capacity, main column condensing capacity and sour-water
condensing capacity.
c. Greater than 9+%Same as in item b, along with targeted
recycle (LPG and light naphtha) and may also wish to consider
the applicability of new maximum propylene technology. This
represents the ultimate propylene production scenario and will
require reconfiguration of the gas concentration unit to facilitate
use of the targeted recycle. The new propylene technology will
require extensive reactor/regenerator section modifications since
a second reactor will be added.
European refiner. This refiner operates a stacked configuration FCC unit that was designed in 1960 for low conversion
of VGO. This unit was the subject of a subsequent technology
upgrade and feed capacity revamps over the last 40 years. In the
late 1990s, the refiner commissioned a hydrocracker, resulting in
a higher quality feed. Subsequently, an FCC revamp was commissioned to handle the higher conversion and increased yield of
propylene associated with better quality feed.
Prior to the feed quality change and unit revamp, the unit
yielded approximately 4.5 wt% propylene, and a marginal increase
was expected with the hydrogen-rich feed. A major revamp was
conducted as a high-conversion enabler. The scope of the revamp
included adding a new riser separation system with improved
feed distributors. This revamp enabled a conversion increase that
resulted in a 4 wt% increase in propylene yield.
HYDROCARBONPROCESSING September 2011
SpecialReport
Refining developments
The refiner is considering further propylene production
increases in conjunction with the catalyst manufacturer that
would produce a propylene yield of nearly 13 wt%. This exemplifies increases in propylene yield that can be achieved as a result
of FCC technology upgrades that enable effective increases in
conversion and improvements to selectivities, a tuned catalyst
system and feed quality improvements.
Other refiner. A refiner replaced its 1940s thermal catalytic
cracking (TCC) unit reaction section with a new high propylene
FCC process so that it could substantially increase its propylene
production through a simultaneous reactor-regenerator technology upgrade and a feed rate increase. Although a total reactorregenerator replacement was required, the product-recovery section was revamped for higher propylene yield and recovery, and
a propylene-recovery unit was installed. It was commissioned
to produce 140,000 metric tpy of polymer-grade propylene.
The new propylene-focused FCC unit was a major revamp of an
obsolete cracking technology that substantially increased polymergrade propylene production. This refiner has achieved more than
16 wt% propylene using an Arabian Light VGO.
Existing FCCs can be, and have been, converted for operations
at or near high propylene FCC conditions. Recently, a newer FCC
operating with enhanced LPG yields revamped its reactor section
Table 1. Spent catalyst recycle impact on unit heat
balance
Base
Base +
reactor process
Reactor temp, F
990
Coke, wt%
5.3
5.2
1,234
1,301
Regenerator temp, F
Cat/oil ratio (Rx-Reg), lb/lb
Cat/oil ratio (Riser), lb/lb
Delta coke, wt%
Fig. 7
990
10.7
8.0
8.1
15.7
0.50
0.65
Latest maximum propylene technology configuration.
to emulate the new high propylene FCC operations. As a result
of this technology upgrade, this unit achieved an 18% increase in
its propylene yield.
The new high propylene FCC technology utilizes low partial pressure, high reactor temperature, a ZSM-5 catalyst system
and features spent catalyst recycle technology. The spent catalyst
recycle technology recycles carbonized, active catalyst from the
stripper to the riser mix zone where it is mixed with regenerated
catalyst. Since the recycled catalyst is heat balance neutral, the
spent catalyst recycle can facilitate a significant increase in the
riser catalyst-to-oil ratio. This technology also helps to suppress
the riser inlet temperature, which in turn reduces dry gas yields,
and the higher catalyst-to-oil ratio contributes associated with its
operation to higher conversion. It allows the catalyst/oil ratio to be
increased well beyond typical limits imposed by a traditional FCC
heat balance. This enables a higher ZSM-5 content in the riser at
any specific ZSM-5 concentration in the circulating equilibrium
catalyst inventory. Furthermore, applying the spent catalyst technology with a ZSM-5 enhanced catalyst system works to improve
the catalysts effectiveness, thus increasing conversion of light
naphtha olefin and selectivity from the catalyst-to-oil increase.
More on spent catalyst recycle technology. This technology can be used as a revamp option and can produce similar
benefits including selectivity improvement and dry-gas management. In addition, because of its heat balance neutral effect, the
net effect of the spent catalyst recycle is a delta coke increase that
manifests as a regenerator temperature increase. This can prove to
be invaluable for refiners processing severely hydrotreated feeds
or is operating at a very low delta coke. Symptomatic of low delta
coke operation is after burn and elevated carbon monoxide (CO)
in flue gas that may require the elevated excess oxygen, an inefficient practice, and the use of CO promoter. Table 1 summarizes
actual pre/post data for an FCC unit where the spent catalyst
recycle technology was added to address the low regenerator temperature. Since its 2005 commercialization, the technology is
operating in six units and is being designed or is in construction
for an additional 12 units.
Commercial application. Because propylene production
is equilibrium limited, recycling higher molecular weight olefins
can be used as another technique to maximize propylene yields.
The linking of reaction equilibrium concepts with reactor and
regenerator technologies results in the latest maximum propylene
FCC technology. This latest technologyhighest-yield propylene
FCC processuses a multi-stage reactor system comprising a
primary hydrocarbon feedstock reaction stage, and a secondary
recycle reaction stage utilizing a common regeneration stage with
continuous circulation of fluidized catalyst between both reactor stages and the regeneration stage. The reactor/regenerator
section is shown in Fig. 7. This system uses two reaction stages
primarily to overcome equilibrium limitations to propylene yield
and selectivity, and secondarily to maximize product flexibility.
For propylene production relative to the severity/feed quality
continuum, the highest-yield technology is represented at the far
right side of Fig. 8 for those refiners that choose to maximize the
production of propylene.
This technology extends propylene yields to greater than 20
wt%. This substantial shift is achieved by the second reactor that
recracks C4+ olefins to propylene. The second riser can be part
of a major revamp, but plot plan considerations require case by
HYDROCARBONPROCESSING September 2011
Refining developments
greatest yield of propylene per unit cost of production. It can provide exceptional performance through the reduction of non-reactive
diluents from the second-stage feedstock, which consists of only
convertible species or those that participate in the equilibrium shift.
In a comparative study of 500,000 metric tpy propylene
(50,000 bpsd fresh feed) units, application of the reactor technology required 12% less capital and 7% less operating cost per
unit of propylene relative to a comingled product recovery system
design. The lower expenses were due to decreased equipment sizes
and the associated energy consumption. Moreover, the reactor
technology produces a net reactor propylene yield in excess of 20
wt%, which exceeds the capability of currently available traditional
FCC technologies.
REFINING/PETROCHEMICAL INTEGRATION OPTIONS
The FCC refining community will be faced with many interesting challenges over the next few decades. A shrinking forecasted US gasoline demand, coupled with potential economic
and legislative factors will likely reshape the future for refining
conversion. This will leave US refiners with the challenge of how
to best utilize the expected open capacity while preserving the
fixed asset base. As propylene demand grows, refiners can leverage
their FCC technology through implementation of the concepts
outlined in this article.
Propylenes remarkable demand growth requires new technologies to capture growth opportunities. Until recently, refiners were
able to capture incremental shifts that had been met with success for
meeting local demand. The first principles for propylene production
from an FCC unit lend well to understanding the propylene potential, reactor conditions and catalyst selection for achieving incremental in propylene production shifts. In particular, the influence
of equilibrium and the introduction of equilibrium manipulation
augment these first principles. Potential propylene yields in excess
of current practice and convention underscore the necessity for new
technology offerings that allow the refiner to achieve propylene
yields well in excess of the current commercial experience. HP
ACKNOWLEDGMENT
Revised and updated from an earlier presentation at the 2011 NPRA Annual
Meeting, March 2122, 2011, San Antonio, Texas.
Riser pilot plant C4= conversion
C4 LCN recycle blend
C4= conversion, wt %
case evaluation. This latest technology provides the ability to
deliver the maximum propylene yield from the conversion of
traditional FCC feeds. It can be the ultimate solution to those
refiners whose primary objective from their FCC has shifted to
maximize propylene production.
The critical technology features of the latest maximum
propylene process technology are:
Maximum riser containment. Each reactor riser is designed
with its own proprietary riser termination device (RTD) and
high-flux stripper to minimize post-riser vapor residence time.
The post-riser reactions that occur at high reactor temperatures
that can favor propylene production are accompanied by non
selective gas yields and the undesirable hydrogen transfer of olefins
to alkanes.
High catalyst to oil riser reactors. The reactors apply
tightly contained riser reactor systems that operate at a very high
(1530) catalyst-to-oil ratio. Riser reactors were chosen over
fluidized-bed reactors to minimize dilute-phase dry-gas formation
and minimize hydrogen transfer reactions that are promoted by
the extensive backmixing of fluidized bed reactors.
Second-stage riser cracking. The spent catalyst recycle
reactor technology allows the second riser catalyst-to-oil ratio
to increase beyond typical limits imposed by heat balance. This
enables a higher ZSM-5 content in the riser at any specific ZSM-5
concentration in the circulating equilibrium catalyst inventory.
Fig. 9 shows the second riser butene conversion and propylene
yield increase with C/O ratio well beyond the 59 available from
a traditional reactor configuration (without reactor technology).
This increases conversion per pass and decreases recycle in the unit
against a specific propylene production target, and enables direct
conversion of butenes to propylene in the second riser without an
intermediate step of polymerization.
Product recovery and targeted recycle. Effluent from each
reactor is routed to independent main columns and partially integrated gas concentration. The first stage reactor effluent is routed
to a standard main column with LCO, HCO and slurry products.
Naphtha and lighter material are taken overhead to an
enhanced absorption based product recovery system. This
recovery system recovers propylene and produces a superheated
C4-light naphtha stream that is feed to the second stage reactor.
The second stage reactor effluent is quenched in a small column
that preheats the fresh feed. The unconverted naphtha and C4reaction products are routed to a depropanizer and debutanizer.
This multi-stage reactor and product separation system can be
the most capital selective design; this configuration produces the
Conventional FCC
Enhanced propylene FCC
High propylene FCC
SpecialReport
20 C/O
Higher conversion at
constant ZSM-5
achieved with spent
catalyst recycle
5 C/O
Latest propylene FCC technology
0
Fig. 8
10
15
Propylene yield, wt-% FF
20
Mapping new reactor technology on the propylene
continuum.
25
ZSM-5, wt%
Fig. 9
New reactor process contribution to butene conversion.
Article copyright 2011 by Gulf Publishing Company. All rights reserved. Printed in U.S.A.
Not to be distributed in electronic or printed form, or posted on a website, without express written permission of copyright holder.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Hookah Bar Business PlanDocument34 pagesHookah Bar Business PlanAbdelkebir LabyadNo ratings yet
- Career Decision-Making Difficulties QuestionnaireDocument2 pagesCareer Decision-Making Difficulties Questionnaireapi-251146669No ratings yet
- 8098 pt1Document385 pages8098 pt1Hotib PerwiraNo ratings yet
- Product Specification PDFDocument2 pagesProduct Specification PDFTato FloresNo ratings yet
- Kx15dtam PDFDocument2 pagesKx15dtam PDFTato FloresNo ratings yet
- 1427071-6 Product Specification PDFDocument2 pages1427071-6 Product Specification PDFTato FloresNo ratings yet
- 1375055-2 Product Specification PDFDocument3 pages1375055-2 Product Specification PDFTato FloresNo ratings yet
- WD Purple 8TBDocument2 pagesWD Purple 8TBAGNo ratings yet
- BHGE Upstream DrillingDocument8 pagesBHGE Upstream DrillingTato FloresNo ratings yet
- Datasheet of DS-7600NI-Q2 P NVR PDFDocument3 pagesDatasheet of DS-7600NI-Q2 P NVR PDFEdesio SilvaNo ratings yet
- KX15DTDocument2 pagesKX15DTTato FloresNo ratings yet
- Datasheet of DS-2CD1023G0E-I V5.5.70 20180730Document4 pagesDatasheet of DS-2CD1023G0E-I V5.5.70 20180730Ashok AeroNo ratings yet
- 2111037-1 Product Specification PDFDocument2 pages2111037-1 Product Specification PDFTato FloresNo ratings yet
- Ds-7200Hqhi-K1 Series Turbo HD DVR: Features and FunctionsDocument3 pagesDs-7200Hqhi-K1 Series Turbo HD DVR: Features and FunctionsTato FloresNo ratings yet
- Product Specification PDFDocument2 pagesProduct Specification PDFTato FloresNo ratings yet
- Ejemplo Dos Fases PDFDocument3 pagesEjemplo Dos Fases PDFTato FloresNo ratings yet
- Buy Now To Create PDF Without Trial Watermark!!: Linear Program - Original Data Linear Program - Original DataDocument3 pagesBuy Now To Create PDF Without Trial Watermark!!: Linear Program - Original Data Linear Program - Original DataTato FloresNo ratings yet
- Diagrama de Flujo Completo PPDocument3 pagesDiagrama de Flujo Completo PPTato FloresNo ratings yet
- Diagrama de Flujo Completo PP PDFDocument3 pagesDiagrama de Flujo Completo PP PDFTato FloresNo ratings yet
- Lean Manufacturing: Cut Costs and Improve FlowDocument54 pagesLean Manufacturing: Cut Costs and Improve FlowTato FloresNo ratings yet
- Buy Now To Create PDF Without Trial Watermark!!: Linear Program - Original Data Linear Program - Original DataDocument3 pagesBuy Now To Create PDF Without Trial Watermark!!: Linear Program - Original Data Linear Program - Original DataTato FloresNo ratings yet
- IOCL's Polypropylene Plant Overview and Production ProcessDocument98 pagesIOCL's Polypropylene Plant Overview and Production ProcessTato FloresNo ratings yet
- Spheripol PlantDocument17 pagesSpheripol PlantTato Flores100% (1)
- Roland Berger Global Petrochemicals 20121113Document15 pagesRoland Berger Global Petrochemicals 20121113Sankar SasmalNo ratings yet
- Vision2010 216 PPTDocument38 pagesVision2010 216 PPTTato FloresNo ratings yet
- Technology Economics Polypropylene Via Gas Phase ProcessDocument78 pagesTechnology Economics Polypropylene Via Gas Phase ProcessTato Flores0% (5)
- EthyleneDocument10 pagesEthyleneTato FloresNo ratings yet
- Steve Global Olefin September 2014Document33 pagesSteve Global Olefin September 2014Tato FloresNo ratings yet
- Perú SRL - 28/09/15: Concepto Ptyt Iipp Ifpp Prbe DT DN DE DEC PPC Uf %av Ue Uf %av UeDocument1 pagePerú SRL - 28/09/15: Concepto Ptyt Iipp Ifpp Prbe DT DN DE DEC PPC Uf %av Ue Uf %av UeTato FloresNo ratings yet
- ReadmeDocument2 pagesReadmeAndres Flores GutierrezNo ratings yet
- Steve Global Olefin September 2014Document33 pagesSteve Global Olefin September 2014Tato FloresNo ratings yet
- Polypropylne Expand On Consumer NeedsDocument53 pagesPolypropylne Expand On Consumer NeedsTato Flores100% (1)
- Coiculescu PDFDocument2 pagesCoiculescu PDFprateek_301466650No ratings yet
- Purpose and Types of Construction EstimatesDocument10 pagesPurpose and Types of Construction EstimatesAisha MalikNo ratings yet
- Guidelines: For Submitting A Candidature To OrganiseDocument19 pagesGuidelines: For Submitting A Candidature To OrganiseDan ZoltnerNo ratings yet
- SECTION 26. Registration of Threatened and Exotic Wildlife in The Possession of Private Persons. - NoDocument5 pagesSECTION 26. Registration of Threatened and Exotic Wildlife in The Possession of Private Persons. - NoAron PanturillaNo ratings yet
- Life Member ListDocument487 pagesLife Member Listpuiritii airNo ratings yet
- Important PIC'sDocument1 pageImportant PIC'sAbhijit SahaNo ratings yet
- Tugas Kelompok 3 Bahasa InggrisDocument5 pagesTugas Kelompok 3 Bahasa InggrisAditya FrediansyahNo ratings yet
- 1 292583745 Bill For Current Month 1Document2 pages1 292583745 Bill For Current Month 1Shrotriya AnamikaNo ratings yet
- City of Watertown Seasonal Collection of Brush and Green WasteDocument3 pagesCity of Watertown Seasonal Collection of Brush and Green WasteNewzjunkyNo ratings yet
- ECC Ruling on Permanent Disability Benefits OverturnedDocument2 pagesECC Ruling on Permanent Disability Benefits OverturnedmeymeyNo ratings yet
- Fortianalyzer v6.2.8 Upgrade GuideDocument23 pagesFortianalyzer v6.2.8 Upgrade Guidelee zwagerNo ratings yet
- People v. Romorosa y OstoyDocument12 pagesPeople v. Romorosa y OstoyArjay ElnasNo ratings yet
- MODULE 3 LeadershipDocument25 pagesMODULE 3 LeadershipCid PonienteNo ratings yet
- Flexible Learning Part 1Document10 pagesFlexible Learning Part 1John Lex Sabines IgloriaNo ratings yet
- AN ORDINANCE ESTABLISHING THE BARANGAY SPECIAL BENEFIT AND SERVICE IMPROVEMENT SYSTEMDocument7 pagesAN ORDINANCE ESTABLISHING THE BARANGAY SPECIAL BENEFIT AND SERVICE IMPROVEMENT SYSTEMRomel VillanuevaNo ratings yet
- International Marketing StrategiesDocument19 pagesInternational Marketing StrategiesHafeez AfzalNo ratings yet
- The History of Thoth 1Document4 pagesThe History of Thoth 1tempt10100% (1)
- Test Bank For Goulds Pathophysiology For The Health Professions 5 Edition Karin C VanmeterDocument36 pagesTest Bank For Goulds Pathophysiology For The Health Professions 5 Edition Karin C Vanmetermulctalinereuwlqu100% (41)
- Business and Transfer Taxation Chapter 11 Discussion Questions AnswerDocument3 pagesBusiness and Transfer Taxation Chapter 11 Discussion Questions AnswerKarla Faye LagangNo ratings yet
- A Research Agenda For Creative Tourism: OnlineDocument1 pageA Research Agenda For Creative Tourism: OnlineFelipe Luis GarciaNo ratings yet
- Vedic MythologyDocument4 pagesVedic MythologyDaniel MonteiroNo ratings yet
- Principles of Administrative TheoryDocument261 pagesPrinciples of Administrative TheoryZabihullahRasidNo ratings yet
- Plan Green Spaces Exam GuideDocument8 pagesPlan Green Spaces Exam GuideJully ReyesNo ratings yet
- Decathlon - Retail Management UpdatedDocument15 pagesDecathlon - Retail Management UpdatedManu SrivastavaNo ratings yet
- Engineering Economy 2ed Edition: January 2018Document12 pagesEngineering Economy 2ed Edition: January 2018anup chauhanNo ratings yet
- Tax 2 AssignmentDocument6 pagesTax 2 AssignmentKim EcarmaNo ratings yet
- Apply for Letter of AdministrationDocument5 pagesApply for Letter of AdministrationCharumathy NairNo ratings yet