Professional Documents
Culture Documents
Staphylococcus Mobile Phone
Uploaded by
Thayumanavan ThangaveluCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Staphylococcus Mobile Phone
Uploaded by
Thayumanavan ThangaveluCopyright:
Available Formats
Biocontrol Science, 2016, Vol. 21, No.
2, 9198
Original
Isolation, Identication and Antibacterial Susceptibility of
Staphylococcus spp. Associated with the Mobile Phones of
University Students
KATSUNORI FURUHATA1, NAOTO ISHIZAKI1, KAZUYUKI SOGAWA1,
YASUSHI KAWAKAMI1, SHIN-ICHI LEE1, MASAHIRO SATO2,
1
AND MASAFUMI FUKUYAMA
1
School of Life and Environmental Science, Azabu University Sagamihara, Kanagawa 252-5201, Japan
2
Faculty of Fukuoka Medical Technology, Teikyo University Omuta, Fukuoka 836-8505, Japan
Received 30 June, 2015/Accepted 25 September, 2015
From May 2014 to February 2015, 319 university studentsmale, n=173; female n=146of 18 to
24 years of age who carried mobile phones or computer tablets were selected as subjects.
Staphylococcus spp. were detected in 101 of 319 samples31.7%. In the present study, 11
strains of S. aureus were isolated and identified, not all of which were methicillin-resistant
Staphylococcus aureusMRSA
. Overall, 14 species were identied, with 11 strains10.9%of S.
xylosus being isolated at the highest frequency. Following this were eight strains7.9%of S.
cohnii and seven strains6.9%each of S. capitis and S. haemolyticus. Staphylococcus spp.
isolation was performed with bacterial samples obtained from the mobile phones of 22 specic
subjectsmales, n=12; females, n=10
. Staphylococcus spp. isolation was performed on days -1,
7 and 30 of the experiment. Staphylococcus spp. were positively detected one or more times in 12
subjects54.5%. In one subject8.3%, all three tests were positive. Furthermore, two tests
were positive in three25.0%
. In the eight remaining subjects66.7%Staphylococcus spp. were
detected only once. For the three abovementioned tests, we investigated the pulsed-field gel
electrophoresisPFGEpatterns of the strains derived from the mobile phone and from the
ngers of three subjects in whom the same bacterial species were isolated twice. From the cases
with similarities between strains derived from the ngers and the mobile phones and cases, with
consistency in the strains derived from the mobile phone at different times, commonality was
observed in the strains derived from the fingers and mobile phones along with chronological
uniformity in the strains derived from the mobile phones. A total of 101 Staphylococcus spp.
strains were isolated from mobile phones. According to drug susceptibility tests, 99 strains
98.0%were found to have some degree of resistance to drugsexcluding one strain each of S.
aureus and S. haemolyticus. Among these, the strain that showed the highest level of drug
resistance was one strain1.0%of Staphylococcus spp., which showed resistance to nine drugs.
The strain that showed the second highest level of drug resistance was one strain1.0%of S.
caprea, which showed resistance to seven drugs. In this manner, the drug-resistant tendencies of
Staphylococcus spp. isolated from mobile phones were observed.
Key wordsStaphylococcus spp. / Mobile phone / Antibacterial susceptibility.
The rate of use of mobile phones has increased drastically since the 1990 s, reaching 100% in 2014, such
that we are now living in an era in which practically all
Corresponding author. Tel: +81-42-754-7111, Fax: +81-42754-6215, E-mail : furuhata
aazabu-u.ac.jp
persons own a mobile phone. Among people 18 - 24
years of age, there is an almost 100% usage rate,
regardless of gender.
Studies of mobile phones have been generally centered
on medical treatment locations. The rst studies examined interference with medical devicesHayes et al.,
92
K. FURUHATA ET AL.
1997
; however, in recent years, there have been many
studies of microbial contaminationHassoun et al.,
2004; Brady et al., 2007; Ramesh et al., 2008; Akinyemi
et al., 2009
. In particular, the results of microbiological
studies of the mobile phones used by medical professionals have indicated that these devices are potential
causes of healthcare-associated infectionsBrady et
al., 2009; Ustun and Cihangiroglu, 2012.
Based on this background, in order to understand
the extent to which bacteria on the hands of healthy
individuals migrate to mobile phones, with a focus on
Staphylococcus species that are naturally found on
human skin, we investigated the presence of these
bacteria on mobile phones.
With regard to Staphylococcus spp., methicillin-resistant Staphylococcus aureusMRSAinfection has
been pointed out to be an important social problem
Barrett et al., 1968. MRSA has previously been
detected on mobile phones used in medical treatment
locationsHassoun et al., 2004. We therefore carried
out antibacterial susceptibility tests for Staphylococcus
spp. adhering to mobile phones. Our objective was to
understand the mechanisms underlying the antibacterial
resistance of the strains of Staphylococcus spp.
isolated from the students phones.
MATERIALS AND METHODS
Subjects
From May 2014 to February 2015, 319 university
studentsmale, n=173; female n=146of 18 to 24 years
of age who carried mobile phones or computer tablets
were selected as subjects. In addition, the collection of
samples were performed based on theEthical Guidelines
for Medical and Health Research Involving Human
Subjects:the objectives and the outline of this study
were sufficiently explained to all research subjects
university studentsby oral means, and samples were
obtained only from those who consented.
Isolation and identication
StanCatch 10 mediumNikken Bio Medical Laboratory
co. Ltd., Kyoto, Japanusing Mannitol salt agar with egg
yolks was used. The backside10 cm2of a mobile
phone was lightly put into contact with the medium, and
culturing was performed at 36 for 48 h. Subsequently,
colonies suspected of including Staphylococcus spp.
were sought and extracted, and reisolation was carried
out using new Mannitol salt agar with egg yolksEiken
Chemical Co., Ltd. The colonies were then purified
with nutrient agar medium. After primary identification
with Gram s staining and OF and catalase tests, a status
examination was performed using an API Staph
Staphylococcus identication kitSYSMEX bioMrieux
Co., Ltd., and bacterial strains with identication odds
of 85% or higher were identied. When Staphylococcus
aureus was indicated, a coagulase test using rabbit
plasmaEiken Chemical Co., Ltd.and a DNase test
using DNA agar mediumNissui Pharmaceutical Co.,
Ltd.were performed for verification. For strains for
which the probability of identication was low, genetic
testing was also performed based on the base
sequence of the 16S rRNAapprox. 500bp
Furuhata,
2007.
MRSA identication test
MRSA screening was performed on the strains identified to be Staphylococcus aureus. When MRSA was
isolated via MRSA Screen agar culturing, a latex slide
agglutination test for penicillin binding protein 2 PBP2
was performed using an MRSA Latex Test for PBP2
MRSA-LA; Denka Seiken Co., Ltd.. In addition, we
also attempted to detect the presence of mecA genes
by using PCROliveria and de Lencastre, 2002.
Periodic attempts to isolate Staphylococcus spp.
from mobile phones
One day before the experimentday 0
, the backside
of the mobile phones of 22 subjectsmales, n=12;
females, n=10were wiped with 70% alcohol disinfectant. The phones were subsequently used in a normal
way. Bacterial culturing was then performed and
Staphylococcus spp. isolation was attempted, according
to the methods described above on days 1, 7, and 30.
Pulsed-eld gel electrophoresis
An analysis of the chromosomal DNA restriction
proles in nine test strains was carried out via pulsedfield gel electrophoresisPFGE. Agarose gel blocks
containing genomic DNA were prepared according to
the methods of Rice et al.1999. The test strains were
cultured for 18 h at 37 in Trypticase Soy BrothBecton
Dickinson, MD, USA. Cells of each strain were
suspended in EET buffer100 mM EDTA, 10 mM EGTA
and 10 mM Tris-HCl, pH 8.0, washed twice and resuspended in 300 l of the same buffer. The cell suspensions were mixed with an equal volume of 1.0% SeaKem
Gold AgaroseTakara Bio, Shiga, Japan, dispensed
into a plug mold and allowed to solidify at 4.
The agarose gel blocks were treated with EET buffer
containing 1 mg/ml of lysozyme, 50 g/mL of mutanolysin and 0.05% N-lauroylsarcosine, and incubated at
37. After three h of incubation, the gel blocks were
again placed into EET buffer containing 1 mg/ml of
Proteinase K and 1% SDS, and incubated overnight at
50. The sample gel blocks were washed three times
with TE buffer10 mM Tris-HCl, 1 mM EDTA, pH 8.0.
Each gel block was digested for 5 h with 3 g of Sma
STAPHYLOCOCCUS SPP. FROM MOBILE PHONES
TOYOBO, Osaka, Japanat 30.
The gel blocks were loaded into each well of 1.0%
PFC agarose gelBIO RAD, CA, USA
. The electrophoretic conditions used were based on those reported by
McDougal et al.2003. Electrophoresis was carried
out at 6 V/cm for 21 h with pulse times ranging from 5
to 40 seconds using the CHEF-DR III systemBIO
RAD, CA, USAwith 0.5TBE buffer44.5 mM Tris,
44.5 mM boric acid and 1 mM EDTA, pH 8.0at 14.
The agarose gel was stained with SYBR Green
Lonzaand the band patterns were visualized using a
UV transilluminator. The Lambda Ladder PFG Marker
New England Biolabs Japan, Tokyo, Japanwas used
as a marker.
Susceptibility testing
A total of 13 antibiotics, including ampicillinABPC:
Sigma-Aldrich Co. LLC, oxacillinMPIPC: SigmaAldrich Co. LLC, cefmetazoleCMZ: Daiichi Sankyo
Co., Ltd., latamoxefLMOX: Shionogi & Co., Ltd,
gentamicinGM: Fuji Pharma Co., Ltd., kanamycin
KM: Sigma-Aldrich Co. LLC, streptomycinSM:
Wako Pure Chemical Industries, Ltd., erythromycin
EM: Wako Pure Chemical Industries, Ltd., lincomycin
LCM: Pharmacia & Upjohn, Co., LLC, tetracycline
TC: Wako Pure Chemical Industries, Ltd., chloramphenicolCP: Sigma-Aldrich Co. LLC, norfloxacin
NFLX: Sigma-Aldrich Co. LLCand vancomycin
VCM: Sigma-Aldrich Co. LLC
, were used as test antibiotics, and the minimum inhibition concentrations
MICswere measured according to the method of the
CLSI2006. The results were interpreted using the
CLSIformerly the National Committee for Clinical
Laboratory StandardscriteriaCLSI, 2007.
The testing isolates were smeared in streaks on
Meller-Hinton agarBecton, Dickinson and Company
and then cultured at 36 for 20 h. The developed colonies
were retrieved and subsequently inoculated in MellerHinton brothBecton, Dickinson and Companyand
cultured at 36 for 20 h. The culture medium was
diluted 100-fold to prepare a suspension of approximately 106 CFU/ml, which was inoculated onto an antibiotic susceptibility testing plate using the Microplanter
MIT-PSakuma K.K.. After culturing the bacteria at
36 for 20 h, colonies of 5 or less were classified as
growth-negative, and the MIC was determined.
RESULTS
Staphylococcus spp. isolated from the surface of
mobile phones
TABLE 1 shows the results for Staphylococcus spp.
and S. aureus detection from the mobile phones according
to gender. Staphylococcus spp. were detected in
93
TABLE 1Positive isolation rates of Staphylococcus spp.
and S. aureus from mobile phones.
Origin
Number of
samples
Male
173
Positive for
Staphylococcus spp.
%
5732.9
Positive for
S.aureus
%
42.3
Female
146
4430.1
74.8
Total
319
10131.7
113.4
: Number of samples
TABLE 2Staphylococcus spp. identied from mobile phone
analysis.
Species
Origin
Male
Female
Total
S. aureusMSSA
46.9
716.3 1110.9
S. xylosus
712.1
49.3
1110.9
S. cohnii
610.3
24.7
87.9
S. capitis
58.6
24.7
76.9
S. haemolyticus
712.1
00
76.9
S. saprophyticus
23.4
37.0
55.0
S. sciuri
46.9
12.3
55.0
S. caprea
00
49.3
44.0
S. warneri
35.2
12.3
44.0
S. epidermidis
23.4
12.3
33.0
S. hominis
11.7
24.7
33.0
S. arlettae
00
12.3
11.0
S. pasteuri
11.7
00
11.0
S. simulans
00
12.3
11.0
Staphylococcus spp. 1627.6 1432.6 3029.7
Total
58100.0 43100.0101100.0
: Number of strains:%
samples obtained from 101 of 319 mobile phones
31.7%. Among these, 57 of 173 samples32.9%
came from the mobile phones of male students, and 44
of 146 samples30.1%came from the mobile phones
of female students. Therefore, no marked gender difference was observed. S. aureus was detected in no more
than 11 samples3.4%, four samples2.3%from
male students and seven samples4.8%from female
students.
TABLE 2 summarizes the Staphylococcus spp.
isolated and identied from the mobile phones. Overall,
14 species were identified, with S. aureus and S.
xylosus isolated at the highest frequency11 strains
each10.9%, followed by S. cohnii8 strains
7.9%, and seven strains6.9%each of S. capitis
and S. haemolyticus. The other Staphylococcus spp.
94
K. FURUHATA ET AL.
TABLE 3The findings of periodic attempts to isolate
Staphylococcus spp. from mobile phones.
Subject No.
1
Days
1
30
10
11
12
; positive; negative
included S. saprophyticus and S. sciurifive strains
each5.0%, S. caprea and S. warnerifour strains
each4.0%, S. epidermidis and S. hoministhree
strains each3.0%, S. arlettae, S. pasteuri and S.
simulansone strain each1.0%. A tot al of 11
species were identied in samples from male students
S. arlettae, S. caprea and S. simulans were not found
,
with S. haemolyticus and S. xylosus being the most
frequentseven strains each12.1%
, followed by S.
cohniisix strains10.3%, and S. capitisve strains
8.6%. A total of 12 species were identified in
samples from female studentsS. haemolyticus and S.
pasteuri were not identified, with the most frequent
being S. aureusseven strains16.3%, followed by
S. caprea and S. xylosusfour strains each9.3%
each. In this study, not all of the 11 strains of S. aureus
isolated and identied were MRSA.
Periodic attempts to isolate Staphylococcus spp.
from mobile phones
TABLE 3 shows the results of the attempts to isolate
Staphylococcus spp. on experimental days 1, 7, and 30
from the mobile phones of 22 selected subjects. On the
three days of the experiment, Staphylococcus spp.
were positively identified one or more times on the
phones of 12 subjects54.5%. Staphylococcus spp.
were isolated on days 1, 7, and 30 from ve41.7%,
ve41.7%and seven subjects58.3%, respectively,
which shows that the detection rate had increased.
Positive detection was made on all three days in one
subject8.3%
, while three subjects25.0%had two
positive testsone subject on days 1 and 7, and two
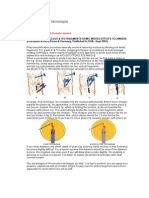
FIG. 1PFGE proles obtained after the Sma digestion of
the nine Staphylococccus spp. strains isolated from ngers of
subjects and mobile phones at different times.
Subjects Nos. 1, 3, and 4
F, ngers; 1, day 1 of the experiment; 7, day 7 of the experiment; 30, day 30 of the experiment.
M, Lambda Ladder PFG Marker.
subjects on days 7 and 30. In the eight remaining
subjects66.7%Staphylococcus spp. were detected
only oncethree subjects on day 1, one subject on day
7 and four subjects on day 30.
PFGE patterns of the strains isolated from the
mobile phones
FIG. 1 shows the PFGE patterns of the strains from
the mobile phones and fingers from three people
Subject Nos. 1, 3 and 4in whom the same bacterial
species were isolated from their mobile phones twice.
S. aureus was isolated from both the fingers and
mobile phone of subject no. 3. Upon an examination of
the PFGE pattern of these strains, the strains isolated
from the mobile phone on days 7 and 30 seemed to be
of the same band pattern, suggesting they were most
likely the same strain. However, differences were
observed at more than seven points between the strain
isolated from the subject s ngers and these two strains
FIG. 1 - left.
S. epidermidis was isolated from both the ngers and
mobile phones in the samples of subject no. 1 on days
1 and 7. Upon an examination of the PFGE pattern in
these strains, although a difference was observed in
one band of the strains isolated from the mobile phone
and fingers on day 1, the band patterns were similar,
suggesting it was most likely the same strain. However,
more than seven points of difference were identied in
STAPHYLOCOCCUS SPP. FROM MOBILE PHONES
TABLE 4Susceptibility of Staphylococcus spp. from mobile
phones to 13 antibiotics.
Antibiotic
Range
g/mL
Peak
MIC50
MIC90
g/mLg/mLg/mL
ABPC
0.125 - 128
0.25
MPIPC
0.031 - 128
0.25
0.25
CMZ
0.25 - 64
LMOX
0.25 - 128
16
0.031
0.063
0.5
0.5
32
GM
0.016 - 8
KM
0.063 - 128
SM
0.125 - 8
EM
0.031 - 128
0.25
0.25
16
LCM
0.031 - 128
0.25
0.25
TC
0.031 - 64
0.063
0.125
0.25
NFLX
CP
0.125 - 32
1 - 64
0.5
0.5
VCM
0.125 - 2
the bands of the strains isolated from the fingers and
phone on day 7FIG. 1 - center.
Moreover, in the same manner, S. epidermidis was
isolated from the fingers and mobile phone of subject
no. 4. Upon an examination of the PFGE pattern in
these strains, although a difference was observed in
one band in the strains isolated from the mobile phone
on day 7 and day 30, band patterns were similar,
suggesting that it was most likely the same strain.
However, differences at seven pointsor morewere
observed in the bands of the strains from the fingers
and phoneFIG. 1 - right.
In this manner, in the case in which similar strains
were derived from the fingers and mobile phoneNo.
1and the cases in which consistent strains were
derived from the mobile phones at different timesNo.
3 and No. 4
, commonality was observed between the
strains of the ngers and the mobile phones, along with
chronological uniformity in the strains isolated from the
mobile phones.
MIC distribution of mobile phone-derived
Staphylococcus spp.
TABLE 4 shows the MIC ranges, peak MIC, 50% MIC
MIC50and 90% MICMIC90values of the 13 antibiotics against the test strains. The ranges of MIC for
CMZ, LMOX, GM, KM, SM, EM, LCM, TC, CP, NFLX
and VCM were wide with a unimodal distribution. In
contrast, ABPC and MPIPC showed the widest range
with a slightly bimodal MIC distribution. In the comparison of the MIC90 values of the tested antibiotics, KM
showed the lowest level of antibacterial activity32 g/
95
mLamong the 14 antibiotics, followed by LMOX and
EM16 g/mL. In contrast, the MIC90 values of the
other antibiotics ranged from 0.25 g/mL to 4 g/mL,
indicating no resistance.
Antibiotic resistance trends
Drug susceptibility testing of the 101 isolated strains
of Staphylococcus spp. revealed that 99 strains98.0%
showed some degree of drug resistance. Only two
strainsS. aureus and S. haemolyticusshowed no
resistance.
TABLE 5 shows the resistance patterns of 46 strains
45.5%that demonstrated resistance to two drugs or
more; a wide range of resistance patterns was observed.
Among these, the strain that showed the highest level of
drug resistance was one strain1.0%of Staphylococcus
spp. which showed resistance to nine drugsABPC/
MPIPC/CMZ/LMOX/KM/EM/LCM/NFLX/TC, followed
by one strain1.0%of S. caprea, which showed resistance to seven drugsABPC/MPIPC/LMOX/KM/EM/
LCM/NFLX. Two strains2.0%, S. xylosus and S.
haemolyticus showed resistance to ve drugsABPC/
MPIPC/EM/LCM/CP and MPIPC/LMOX/EM/LCM/
NFLX, respectively.
Five strains5.0%demonstrated resistance to four
drugsS. saprophyticus and Staphylococcus spp. to
ABPC/MPIPC/LMOX/EM, one strain of S. xylosus to
ABPC/MPIPC/EM/LCM, and one strain of
Staphylococcus spp. to ABPC/MPIPC/LMOX/LCM and
another to ABPC/KM/EM/NFLX. Additionally, 15
strains14.9%were resistant to three drugsfour
strains of S. saprophyticus and one strain each of S.
cohnii and S. xylosus to ABPC/MPIPC/LMOX, two strains
each of S. cohnii and S. xylosus to ABPC/MPIPC/LCM,
one strain of S. cohnii to ABPC/MPIPC/KM, one strain
of S. haemolyticus to ABPC/LMOX/TC, one strain of S.
caprea to ABPC/KM/NFLX, one strain of S. epidermidis
to ABPC/EM/CP, and one strain of Staphylococcus spp.
to MPIPC/LMOX/NFLX. The drug patterns with the
relevant bacterial species and strain counts for the twodrug-resistant strains are as shown in TABLE 5.
DISCUSSION
The use of mobile phones has spread rapidly in recent
years. In the present study, we investigated the adhesion of Staphylococcus spp., a resident of the skin ora,
to mobile phones based on the hypothesis that mobile
phones may be sources of infection, such as healthcare-associated infections, etc.Yamada et al., 2010;
Morioka et al., 2011. Although many such investigations of contamination have been reported in recent
yearsBraddy et al., 2005; Karabay et al., 2007; Brady
et al., 2011; Selim and Abaza, 2015, only one of these
96
K. FURUHATA ET AL.
TABLE 5Multi-antimicrobial resistance patterns of Staphylococcus spp. from mobile phones.
No. of antibiotic
9
7
5
Resistance pattern
ABPC/ MPIPC/ CMZ/
EM/
LMOX/
KM/
NFLX/
TC
ABPC/ MPIPC/ LMOX/
KM/
EM/
LCM/
LCM/
NFLX
ABPC/ MPIPC/
EM/
LCM/
CP
MPIPC/ LMOX/
EM/
LCM/
NFLX
Speices
No. of isolates
Staphylococcus spp.
11.0
S. caprea
11.0
S. xylosus
S. haemolyticus
Subtotal
22.0
ABPC/ MPIPC/ LMOX/
EM
S. saprophyticus
Staphylococcus spp.
ABPC/ MPIPC/ LMOX/
LCM
Staphylococcus spp.
ABPC/ MPIPC/
EM/
LCM
S. xylosus
ABPC/
EM/
NFLX
Staphylococcus spp.
KM/
Subtotal
55.0
ABPC/ MPIPC/ LMOX
S. saprophyticus
S. cohnii
S. xylosus
ABPC/ MPIPC/
KM
S. cohnii
ABPC/ MPIPC/
LCM
S. cohnii
S. xylosus
ABPC/ LMOX/
TC
S. haemolyticus
S. caprea
S. epidermidis
Staphylococcus spp.
ABPC/
KM/
ABPC/
EM/
MPIPC/ LMOX/
NFLX
CP
NFLX
Subtotal
1514.9
ABPC/ MPIPC
S. cohnii
ABPC/ LMOX
S. capitis
S. epidermidis
S. hominis
S. sciuri
ABPC/
SM
S. sciuri
ABPC/
EM
S. sciuri
Staphylococcus spp.
S. aureus
S. warneri
S. haemolyticus
Staphylococcus spp.
S. simulans
Staphylococcus spp.
2
MPIPC/ LMOX
MPIPC/
KM
MPIPC/
EM
S. pasteuri
MPIPC/
TC
S. haemolyticus
EM/
TC
Staphylococcus spp.
Subtotal
2221.8
Total
4645.5
n101
STAPHYLOCOCCUS SPP. FROM MOBILE PHONES
reports provides a detailed identication that includes a
breakdown of coagulase-negative staphylococciCNS
.
In the present study, Staphylococcus spp. were isolated
from 31.7% of the mobile phones, and 14 species were
identified. S. aureus and S. xylosus were the species
most frequently identified10.9%, followed by S.
cohnii7.9%and S. capitis and S. haemolyticus at
6.9%. Moreover, none of the 11 strains of S. aureus
isolated in this study were MRSA. In contrast, the
reports of Brady et al.2005and Jayalakshmi et al.
2008found that MRSA was isolated from mobile
phones at rates of 1.9% and 2.7%, respectively. In
these previously mentioned reports, the subjects of the
investigation were patients and healthcare staff
members. We hypothesize that our results differed from
those of previous studies because we targeted healthy
university students.
Another purpose of this investigation was to conrm
the transmission of resident flora between the fingers
and mobile phones and/or the chronological uniformity
of the isolated strains. Therefore, Staphylococcus spp.
were isolated and identied from the mobile phones of
22 specic participants on days 1, 7, and 30. On these
three designated days, Staphylococcus spp. were
isolated on all three days from one participant8.3%.
Moreover, ora were isolated on two days from three of
the participants25.0%. Among these cases, the
PFGE patterns of the strains from mobile phones and
ngers, focusing on the same strain type isolated from
the mobile phones of three participants in two trials,
were individually compared. A pattern of consistency
was observed in the strains derived from the mobile
phones at different times, while commonality was
observed in the strains from the fingers and mobile
phones, along with chronological uniformity in the
strains from the mobile phones. A report by Borer et al.
2005found that when the migration pattern was
compared using PFGEin the same manner as in our
study, a consistent pattern of multidrug-resistant
Acinetobacter spp. was observed in the strains derived
from mobile phones and ngers, thereby conrming the
transmission of bacteria between these two sources. In
addition, Kanayama et al.2012reported that the
gene patterns of MRSA strains isolated from ngers and
mobile phones were the same. The similar results of
these reports to our own lead us to believe that resident
flora are transmitted between the fingers and mobile
phones.
Most reports related to the drug susceptibility of
Staphylococcus spp. have focused on S. aureusHong
et al., 2015; Krewer et al., 2015; Riva et al., 2015. No
reports have focused on specic Staphylococcus spp.
In the present study, drug susceptibility testing was
carried with the 101 strains isolated from mobile phones
97
in order to investigate the degree of drug resistance of
Staphylococcus spp. Ninety-nine strains98.0%showed
resistance to at least one test drugone strain each of
S. aureus and S. haemolyticus showed no drug resistance. The strain with the highest resistance was one
strain of Staphylococcus spp., which was resistant to
nine drugsABPC/MPIPC/CMZ/LMOX/KM/EM/LCM/
NFLX/TC. Subsequently, one strain of S. caprea
showed resistance to seven drugsABPC/MPIPC/
LMOX/KM/EM/LCM/NFLX
. In this manner, a tendency
for drug resistance was observed in the Staphylococcus
spp. isolated from mobile phones. Therefore, attention
must be paid to future trends in drug resistance. We also
plan to analyze genes related to resistance with respect
to these species.
REFERENCES
Akinyemi, K. O., Atapu, A. D., Adetona, O. O., and Coker, A. O.
2009The potential role of mobile phones in the spread of
bacterial infections. J. Infect. Dev. Ctries., 3, 628-632.
Al-Abdalall, A. H.2010Isolation and identification of
microbes associated with mobile phones in Dammam in
eastern Saudi Arabia. J. Family Community Med., 17, 11-14.
Barrett, F. F., McGehee, R. F. Jr., and Finland, M.1968
Methicillin-resistant Staphylococcus aureus at Boston city
hospital. Bacteriologic and epidemiologic observations. N.
Engl. J. Med., 279, 441-448.
Borer, A., Gilad, J., Smolyakov, R., Eskira, S., Peled, N., Porat,
N., Hyam, E., Treer, R., Rissenberg, K., and Schlaeffer, F.
2005Cell phones and Acinetobacter transmission. Emerg.
Infect. Dis., 11, 1160-1161.
Braddy, C. M., and Blair, J. E.2005Colonization of personal
digital assistants used in a health care setting. Am. J. Infect.
Control., 33, 230-232.
Brady, R. R. W., Wasson, A., Stirling, I., McAllister, C., and
Damani, N. N.2005Is your phone bugged? The incidence
of bacteria known to cause nosocomial infection on healthcare workers mobile phones. J. Hosp. Infect., 62, 123-125.
Brady, R. R., Fraser, S. F., Dunlop, M. G., Paterson-Brown, S.,
and Gibb, A. P.2007Bacterial contamination of mobile
communication devices in the operative environment. J.
Hosp. Infect., 66, 397-398.
Brady, R. R. W., Verran, J., Damani, N. N., and Gibb, A. P.
2009Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J. Hosp. Infect., 71,
295-300.
Brady, R. R., Hunt, A. C., Visvanathan, A., Rodrigues, M. A.,
Graham, C., Rae, C., Kalima, P., Paterson, H. M., and
Gibb, A. P.2011Mobile phone technology and hospitalized patients: a cross-sectional surveillance study of bacterial colonization, and patient opinions and behaviours. Clin.
Microbiol. Infect., 17, 830-835.
Clinical and Laboratory Standard Institute2006Methods
for dilution antimicrobial susceptibility tests for bacteria that
grow aerobically; approved standard-7th edition. M7-A7.
CLSI, Wayne, PA.
Clinical and Laboratory Standard Institute2007Performance
standards for antimicrobial susceptibility testing; 17th information supplement. M100-S17. CLSI, Wayne, PA.
French, G., Rayner, D., Branson, M., and Walsh, M.1998
Contamination of doctors and nurses pens with nosoco-
98
K. FURUHATA ET AL.
mial pathogens. Lancet, 351, 213.
Furuhata, K., Kato, Y., Goto, K., Saitou, K., Sugiyama, J., Hara,
M., and Fukuyama, M.2007Identification of yellowpigmented bacteria isolated from hospital tap water in Japan
and their chlorine resistance. Biocontrol Sci., 12, 39-46.
Hassoun, A., Vellozzi, E. M., and Smith, M. A.2004
Colonization of personal digital assistants carried by healthcare professionals. Infect. Control Hosp. Epidemiol., 25,
1000-1001.
Hayes, D. L., Wang, P. J., Reynolds, D. W., Mark Estes , N.
A., Grifth, J. L., Steffens, R. A., Carlo, G. L., Findlay, G. K.,
and Johnson, C. M.1997Interference with cardiac pacemakers by cellular telephones. N. Engl. J. Med., 336,
1473-1479.
Hong, J., Kim, Y., Kim, J., Heu, S., Km, Se-ri, Kim, K-P., and
Roh, E.2015Genetic diversity and antibiotic resistance
patterns of Staphylococcus aureus isolated from leaf vegetables in Korea. J. Food Sci., Vol.0, Nr.0, DOI: 10.1111/
1750-3841.12909.
Jayalakshmi, J., Appalaraju, B., and Usha, S.2008
Cellphones as reservoirs of nosocomial pathogens. J.
Assoc. Physicians India., 56, 388-389.
Kanayama, A., Kobayashi, I., Yoshizawa, S., Tateda, K., and
Kaneko, A.2012Surface bacterial contamination of
mobile phones leading to contamination of the hands of
healthcare workers. American Society for Microbiology,
112nd General Meeting. San Francisco, USA.
Karabay, O., Kocoglu, E., and Tahtaci, M.2007The role of
mobile phones in the spread of bacteria associated with
nosocomial infections. J. Infect. Developing Countries, 1,
72-73.
Krewer, C. da C., Amanso, E. S., Gouveia, G. V., Souza, R.
de L., da Costa, M. M., and Mota, R. A.2015Resistance
to antimicrobials and biofilm formation in staphylococcus
spp. isolated from bovine mastitis in the Northeast of Brazil.
Trop. Anim. Health Prod., 47, 511-518.
McDougal, L. K., Steward, C. D., Killgore, G. E., Chaitram, J. M.,
McAllister, S. K., and Tenover, F. C.2003Pulsed-eld gel
electrophoresis typing of oxacillin-resistant Staphylococcus
aureus isolated from the United States: establishing a national
database. J. Clin. Microbiol., 41, 5113-5120.
Morioka, I., Tabuchi, Y., Takahashi, Y., Oda, Y., Nakai, M.,
Yanase, A., and Watazu, C.2011Bacterial contamination
of mobile phones shared in hospital wards and the
consciousness and behavior of nurses about biological
cleanlinessin Japanese
. Jpn. J. Hyg., 66, 115-121.
Oliveria, D. C., and de Lencastre, H.2002Multiplex PCR
strategy for rapid identication of structural types and variants
of the mec element in methicillin-resistant Staphylococccus
aureus. Antimicrob. Agents. Chemother., 46, 2155-2161.
Ramesh, J., Carter, A. O., Campbell, M. H., Gibbons, N.,
Powlett, C., Moseley, H. Sr., Lewis, D., and Carter, T.2008
Use of mobile phones by medical staff at Queen Elizabeth
Hospital, Barbados: evidence for both benet and harm. J.
Hosp. Infect., 70, 160-165.
Rice, D. H., Mcmenamin, K. M., Pritchett, L. C., Hancock, D. D.,
and Besser, T. E.1999Genetic subtyping of Eschericha coli
O157 isolates from 41 Pacific Northwest USA cattle farms.
Epidemiol. Infect., 122, 479-484.
Riva, A., Borghi, E., Cirasola, D., Colmegna, S., Borgo, F.,
Amato, E., Pontello, M. M., and Morace, G.2015
Methicillin-resistant Staphylococcus aureus in raw milk:
prevalence, SCC mec typing, enterotoxin characterization,
and antimicrobial resistance patterns. J. Food Prot., 78,
1142-1146.
Selim, H. S., and Abaza, A. F.2015Microbial contamination
of mobile phones in a health care setting in Alexandria,
Egypt. GMS Hyg. Infect. Control., 10, Doc03. DOI: 10.3205/
dgkh000246, URN: urn:nbn:de:0183-dgkh0002461.
Tenover, F. C., Arbeit, R. D., Goering, R. V., Mickelsen, P. A.,
Murray, B. E., Persing, D. H., and Swaminathan, B.1995
Interpreting chromosomal DNA restriction patterns produced
by pulsed-eld gel electrophoresis: criteria for bacterial strain
typing. J. Clin. Microbiol., 33, 2233-2239.
Tenover, F. C., Arbeit, R. D., and Goering, R. V.1997How to
select and interpret molecular strain typing methods for
epidemiological studies of bacterial infection: a review for
healthcare epidemiologists. Infect. Control. Hosp. Epidemiol.,
18, 426-439.
Ustun, C., and Cihangiroglu, M.2012Health care workers
mobile phones: a potential cause of microbial crosscontamination between hospitals and community. J. Occup.
Environ. Hyg., 9, 538-542.
Yamada, K., Sakaguchi, K., Fujiwara, D., Tani, K., and Ogawa,
M.2010Bacterial contamination of personal handy-phone
system in a hospitalin Japanese
. Kankyokansen-shi, 25,
163-166.
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Aritmia LetalDocument53 pagesAritmia LetalMetta SariNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Medical Billing and Coding SpecialistDocument2 pagesMedical Billing and Coding Specialistapi-77786926100% (3)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Pediatric PneumoniaDocument12 pagesPediatric PneumoniaKrystal Migo Denolo ContrerasNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Nosocomial PneumoniaDocument37 pagesNosocomial PneumoniaWinson ChitraNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Teaching Plan For HypertensionDocument5 pagesTeaching Plan For HypertensionMaureen Mae Binohlan100% (1)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Bionic Eye AbstractDocument3 pagesBionic Eye Abstractvamsi28No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Professionals and Practitioners in CounselingDocument20 pagesProfessionals and Practitioners in CounselingJac KieNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Pulse DiagnoseDocument427 pagesPulse DiagnoseSnezana MihajlovicNo ratings yet
- Transcultural NursingDocument32 pagesTranscultural Nursingnovi100% (2)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Oxygen-Induced Hypercapnia in COPD PDFDocument4 pagesOxygen-Induced Hypercapnia in COPD PDFsatyagraha84No ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Acute Respiratory Distress Syndrome (ARDS)Document3 pagesAcute Respiratory Distress Syndrome (ARDS)akish4uNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Pathology Mcqs 2Document6 pagesPathology Mcqs 2Numan Rox100% (5)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- HepatitisDocument8 pagesHepatitisudaybujjiNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Case Report OsteochondromaDocument43 pagesCase Report OsteochondromaFidesha Nurganiah SiregarNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Coombs TestDocument5 pagesCoombs TestMima Fatimah LuthfieNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- What Is Pityriasis Rosea?Document1 pageWhat Is Pityriasis Rosea?andinaNo ratings yet
- Albuterol Pediatric Drug CardDocument2 pagesAlbuterol Pediatric Drug CardAnthonyMedinaNo ratings yet
- Bleeding PregDocument261 pagesBleeding PregElsya ParamitasariNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Woodcutters TechniqueDocument3 pagesWoodcutters TechniqueToo SmallNo ratings yet
- Introduction Sports MassageDocument6 pagesIntroduction Sports MassageHari SetiawanNo ratings yet
- Phobias and FearsDocument7 pagesPhobias and Fearsdanyela44No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- GSRC Trans Resource Guide (2015-2016)Document19 pagesGSRC Trans Resource Guide (2015-2016)Merissa Taylor-Meissner100% (1)
- Strickland PresentationDocument1 pageStrickland Presentationmariopi2495No ratings yet
- KyphosisDocument4 pagesKyphosisclubsanatateNo ratings yet
- Control of Blood Glucose LevelDocument21 pagesControl of Blood Glucose LevelalynnsakinaNo ratings yet
- Severe Vata DisordersDocument3 pagesSevere Vata DisordersAkhila VijayakumarNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Journal Reading Clinicopathological Study of Lichen Amyloidosis - Kartika Mega Utami BouwDocument4 pagesJournal Reading Clinicopathological Study of Lichen Amyloidosis - Kartika Mega Utami BouwmarinNo ratings yet
- Therapeutic Management of Laryngectomy - PDF / KUNNAMPALLIL GEJO JOHNDocument31 pagesTherapeutic Management of Laryngectomy - PDF / KUNNAMPALLIL GEJO JOHNKUNNAMPALLIL GEJO JOHNNo ratings yet
- Oral Manifestations of Connective Tissue Disease (CTDDocument34 pagesOral Manifestations of Connective Tissue Disease (CTDFatin Nabihah Jamil67% (3)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)